Cobimetinib in Pediatric and Young Adult Patients with Relapsed or Refractory Solid Tumors (iMATRIX-cobi): A Multicenter, Phase I/II Study
- PMID: 35715627
- PMCID: PMC9217999
- DOI: 10.1007/s11523-022-00888-9
Cobimetinib in Pediatric and Young Adult Patients with Relapsed or Refractory Solid Tumors (iMATRIX-cobi): A Multicenter, Phase I/II Study
Abstract
Background: The MAPK pathway is an emerging target across a number of adult and pediatric tumors. Targeting the downstream effector of MAPK, MEK1, is a proposed strategy to control the growth of MAPK-dependent tumors.
Objective: iMATRIX-cobi assessed the safety, pharmacokinetics, and anti-tumor activity of cobimetinib, a highly selective MEK inhibitor, in children and young adults with relapsed/refractory solid tumors.
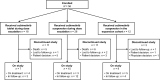
Patients and methods: This multicenter Phase I/II study enrolled patients aged 6 months to < 30 years with solid tumors with known/expected MAPK pathway involvement. Patients received cobimetinib tablet or suspension formulation on Days 1-21 of a 28-day cycle. Dose escalation followed a rolling 6 design. The primary endpoint was safety; secondary endpoints were pharmacokinetics and anti-tumor activity.
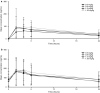
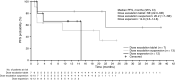
Results: Of 56 enrolled patients (median age 9 years [range 3-29]), 18 received cobimetinib tablets and 38 cobimetinib suspension. Most common diagnoses were low-grade glioma (LGG; n = 32, including n = 12 in the expansion cohort) and plexiform neurofibroma within neurofibromatosis type 1 (n = 12). Six patients (11 %) experienced dose-limiting toxicities (including five ocular toxicity events), which established a pediatric recommended Phase II dose (RP2D) of 0.8 mg/kg tablet and 1.0 mg/kg suspension. Most frequently reported treatment-related adverse events were gastrointestinal and skin disorders. Steady state mean exposure (Cmax, AUC0-24) of cobimetinib at the RP2D (1.0 mg/kg suspension) was ~ 50 % lower than in adults receiving the approved 60 mg/day dose. Overall response rate was 5.4 % (3/56; all partial responses in patients with LGG).
Conclusions: The safety profile of cobimetinib in pediatrics was similar to that reported in adults. Clinical activity was observed in LGG patients with known/suspected MAPK pathway activation. Cobimetinib combination regimens may be required to improve response rates in this pediatric population.
Clinical trial registration: ClinicalTrials.gov NCT02639546, registered December 24, 2015.
© 2022. The Author(s).
Conflict of interest statement
TT reports consultancy fees from F. Hoffmann-La Roche Ltd. HT reports lecture fees from AstraZeneca and Roche. SG reports personal fees from Bayer, EUSA Pharma, and LOXO Oncology. CR reports personal fees from Amgen, BMS, Celgene, Genentech, Inc., Novartis, Pfizer, and F. Hoffmann-La Roche Ltd. DP was an employee of F. Hoffman-La Roche Ltd. at the time of study conduct. LM was an employee of Genentech, Inc. at the time of study conduct. LRP is an employee of F. Hoffmann-La Roche Ltd. SC, KEH, CD, and RB are employees of and stock-holders in F. Hoffmann-La Roche Ltd/Genentech, Inc. All other authors declare no competing interests.
Figures
References
-
- Vitagliano O, Addeo R, D’Angelo V, Indolfi C, Indolfi P, Casale F. The Bcl-2/Bax and Ras/Raf/MEK/ERK signaling pathways: implications in pediatric leukemia pathogenesis and new prospects for therapeutic approaches. Expert Rev Hematol. 2013;6:587–597. doi: 10.1586/17474086.2013.827415. - DOI - PubMed
-
- Shern JF, Chen L, Chmielecki J, Wei JS, Patidar R, Rosenberg M, et al. Comprehensive genomic analysis of rhabdomyosarcoma reveals a landscape of alterations affecting a common genetic axis in fusion-positive and fusion-negative tumors. Cancer Discov. 2014;4:216–231. doi: 10.1158/2159-8290.CD-13-0639. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

