Ovarian carcinosarcoma is a distinct form of ovarian cancer with poorer survival compared to tubo-ovarian high-grade serous carcinoma
- PMID: 35715633
- PMCID: PMC9470739
- DOI: 10.1038/s41416-022-01874-8
Ovarian carcinosarcoma is a distinct form of ovarian cancer with poorer survival compared to tubo-ovarian high-grade serous carcinoma
Abstract
Background: Ovarian carcinosarcoma (OCS) is an uncommon, biphasic and highly aggressive ovarian cancer type, which has received relatively little research attention.
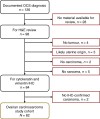
Methods: We curated the largest pathologically confirmed OCS cohort to date, performing detailed histopathological characterisation, analysis of features associated with survival and comparison against high-grade serous ovarian carcinoma (HGSOC).
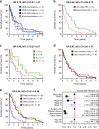
Results: Eighty-two OCS patients were identified; overall survival was poor (median 12.7 months). In all, 79% demonstrated epithelial components of high-grade serous (HGS) type, while 21% were endometrioid. Heterologous elements were common (chondrosarcoma in 32%, rhabdomyosarcoma in 21%, liposarcoma in 2%); chondrosarcoma was more frequent in OCS with endometrioid carcinomatous components. Earlier stage, complete resection and platinum-containing adjuvant chemotherapy were associated with prolonged survival; however, risk of relapse and mortality was high across all patient groups. Histological subclassification did not identify subgroups with distinct survival. Compared to HGSOC, OCS patients were older (P < 0.0001), more likely to be FIGO stage I (P = 0.025), demonstrated lower chemotherapy response rate (P = 0.001) and had significantly poorer survival (P < 0.0001).
Conclusion: OCS represents a distinct, highly lethal form of ovarian cancer for which new treatment strategies are urgently needed. Histological subclassification does not identify patient subgroups with distinct survival. Aggressive adjuvant chemotherapy should be considered for all cases, including those with early-stage disease.
© 2022. The Author(s).
Conflict of interest statement
RLH: consultancy fees from GSK outside the scope of this work. IC: none. MC: none. TR: none. CB: none. CG: CG: grants from AstraZeneca, MSD, BMS, Clovis, Novartis, BerGenBio, Medannexin and Artios; personal fees from AstraZeneca, MSD, GSK, Tesaro, Clovis, Roche, Foundation One, Chugai, Takeda, Sierra Oncology, Takeda and Cor2Ed outside the submitted work; patents PCT/US2012/040805 issued, PCT/GB2013/053202 pending, 1409479.1 pending, 1409476.7 pending and 1409478.3 pending. CSH: none.
Figures



References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

