Geniposide suppresses NLRP3 inflammasome-mediated pyroptosis via the AMPK signaling pathway to mitigate myocardial ischemia/reperfusion injury
- PMID: 35715805
- PMCID: PMC9205109
- DOI: 10.1186/s13020-022-00616-5
Geniposide suppresses NLRP3 inflammasome-mediated pyroptosis via the AMPK signaling pathway to mitigate myocardial ischemia/reperfusion injury
Abstract
Background: NLRP3 inflammasome activation and pyroptosis play a significant role in myocardial ischemia reperfusion injury (MI/RI). Geniposide was reported to show potential therapeutic use for MI/RI with its anti-inflammatory and anti-oxidative properties. However, research on the specific mechanism of geniposide has not been reported.
Methods: The MIRI model of animal was created in male C57BL/6J mice and the hypoxia reoxygenation (H/R) model was established for the in vitro experiments. Neonatal rat ventricular myocytes (NRVMs) and H9c2 cells with knockdown of TXNIP or NLRP3 were used. Geniposide was administered to mice before vascular ligation. HE staining, 2,3,5-triphenyltetrazolium chloride (TTC) staining, echocardiography, oxidative stress and myocardial enzyme detection were used to evaluate the cardioprotective effect of geniposide. Meanwhile, pharmacological approaches of agonist and inhibitor were used to observe potential pathway for geniposide cardioprotective in vitro and in vivo. Moreover, ELISA kits were adopted to detect the levels of inflammatory factors, such as IL-1β and IL-18. The gene and protein expression of NLRP3 and pyroptosis-related factors in heart tissue were performed by RT-PCR, western blotting and immunofluorescence in vivo and in vitro, respectively.
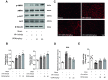
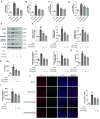
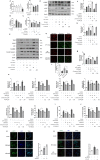
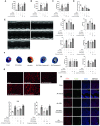
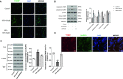
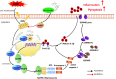
Results: Our results indicate that geniposide can reduce the area of myocardial infarction, improve heart function, and inhibit the inflammatory response in mice after MI/RI. In addition, RT-PCR and western blotting shown geniposide promoting AMPK phosphorylation to activate myocardium energy metabolism and reducing the levels of genes and proteins expression of NLRP3, ASC, N-GSDMD and cleaved caspase-1, IL-1β, IL-18. Meanwhile, geniposide improved NRVMs energy metabolism, which decreased ROS levels and the protein expression of TXNIP and thus suppressed the expression of NLRP3. AMPK antagonist or agonist and siRNA downregulation of TXNIP or NLRP3 were also verify the effect of geniposide against H/R injury. Further research found that geniposide promoted the translocation of TXNIP and reduce the binding of TXNIP and NLRP3.
Conclusions: In our study, geniposide can significantly inhibit NLRP3 inflammasome activation via the AMPK signaling pathway and inhibit pyroptosis of cardiomyocytes in myocardial tissues.
Keywords: AMPK; Geniposide; Myocardial ischemia reperfusion; NLRP3 inflammasome; Pyroptosis.
© 2022. The Author(s).
Conflict of interest statement
The authors have declared that no competing interest exists.
Figures


References
-
- Zeng H, Wang L, Zhang J, Pan T, Yu Y, Lu J, et al. βActivated PKB/GSK-3 synergizes with PKC-signaling in attenuating myocardial ischemia/reperfusion injury potentiation of NRF2 activity: therapeutic efficacy of dihydrotanshinone-I. Acta Pharm Sin B. 2021;11(1):71–88. doi: 10.1016/j.apsb.2020.09.006. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

