Fatty acids role in multiple sclerosis as "metabokines"
- PMID: 35715809
- PMCID: PMC9205055
- DOI: 10.1186/s12974-022-02502-1
Fatty acids role in multiple sclerosis as "metabokines"
Abstract
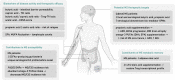
Multiple sclerosis (MS), as an autoimmune neurological disease with both genetic and environmental contribution, still lacks effective treatment options among progressive patients, highlighting the need to re-evaluate disease innate properties in search for novel therapeutic targets. Fatty acids (FA) and MS bear an interesting intimate connection. FA and FA metabolism are highly associated with autoimmunity, as the diet-derived circulatory and tissue-resident FAs level and composition can modulate immune cells polarization, differentiation and function, suggesting their broad regulatory role as "metabokines". In addition, FAs are indeed protective factors for blood-brain barrier integrity, crucial contributors of central nervous system (CNS) chronic inflammation and progressive degeneration, as well as important materials for remyelination. The remaining area of ambiguity requires further exploration into this arena to validate the existed phenomenon, develop novel therapies, and confirm the safety and efficacy of therapeutic intervention targeting FA metabolism.
Keywords: Fatty acid metabolism; Immune; Multiple sclerosis; Neurodegeneration.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




Similar articles
-
Fatty Acid Synthesis in Glial Cells of the CNS.Int J Mol Sci. 2021 Jul 29;22(15):8159. doi: 10.3390/ijms22158159. Int J Mol Sci. 2021. PMID: 34360931 Free PMC article. Review.
-
CNS myelination and remyelination depend on fatty acid synthesis by oligodendrocytes.Elife. 2019 May 7;8:e44702. doi: 10.7554/eLife.44702. Elife. 2019. PMID: 31063129 Free PMC article.
-
Microglia as therapeutic targets for central nervous system remyelination.Curr Opin Pharmacol. 2022 Apr;63:102188. doi: 10.1016/j.coph.2022.102188. Epub 2022 Feb 23. Curr Opin Pharmacol. 2022. PMID: 35219055 Review.
-
Thyroid Hormone Potentially Benefits Multiple Sclerosis via Facilitating Remyelination.Mol Neurobiol. 2016 Sep;53(7):4406-16. doi: 10.1007/s12035-015-9375-z. Epub 2015 Aug 5. Mol Neurobiol. 2016. PMID: 26243185 Review.
-
The Molecular Basis for Remyelination Failure in Multiple Sclerosis.Cells. 2019 Aug 3;8(8):825. doi: 10.3390/cells8080825. Cells. 2019. PMID: 31382620 Free PMC article. Review.
Cited by
-
Utility of an Untargeted Metabolomics Approach Using a 2D GC-GC-MS Platform to Distinguish Relapsing and Progressive Multiple Sclerosis.Metabolites. 2024 Sep 11;14(9):493. doi: 10.3390/metabo14090493. Metabolites. 2024. PMID: 39330500 Free PMC article.
-
Lipid metabolism in type 1 diabetes mellitus: Pathogenetic and therapeutic implications.Front Immunol. 2022 Oct 6;13:999108. doi: 10.3389/fimmu.2022.999108. eCollection 2022. Front Immunol. 2022. PMID: 36275658 Free PMC article. Review.
-
Immuno-nutritional therapy in experimental autoimmune encephalomyelitis: a translational pathway to multiple sclerosis management.Inflammopharmacology. 2025 Jun 17. doi: 10.1007/s10787-025-01804-z. Online ahead of print. Inflammopharmacology. 2025. PMID: 40528137 Review.
-
Exploring Human Brain Metabolism via Genome-Scale Metabolic Modeling with Highlights on Multiple Sclerosis.ACS Chem Neurosci. 2025 Apr 2;16(7):1346-1360. doi: 10.1021/acschemneuro.5c00006. Epub 2025 Mar 17. ACS Chem Neurosci. 2025. PMID: 40091499 Free PMC article.
-
Epigenetic and Mitochondrial Metabolic Dysfunction in Multiple Sclerosis: A Review of Herbal Drug Approaches and Current Clinical Trials.Mol Neurobiol. 2025 Aug;62(8):10045-10090. doi: 10.1007/s12035-025-04868-8. Epub 2025 Apr 3. Mol Neurobiol. 2025. PMID: 40180689 Free PMC article. Review.
References
-
- Olsson T, Barcellos LF, Alfredsson L. Interactions between genetic, lifestyle and environmental risk factors for multiple sclerosis. Nat Rev Neurol. 2016;13:26–36. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

