Spotlight on therapeutic efficiency of mesenchymal stem cells in viral infections with a focus on COVID-19
- PMID: 35715852
- PMCID: PMC9204679
- DOI: 10.1186/s13287-022-02944-7
Spotlight on therapeutic efficiency of mesenchymal stem cells in viral infections with a focus on COVID-19
Abstract
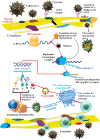
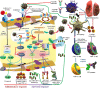
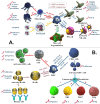
The SARS-COV-2 virus has infected the world at a very high rate by causing COVID-19 disease. Nearly 507 million individuals have been infected with this virus, with approximately 1.2% of these patients being dead, indicating that this virus has been out of control in many countries. While researchers are investigating how to develop efficient drugs and vaccines versus the COVID-19 pandemic, new superseded treatments have the potential to reduce mortality. The recent application of mesenchymal stem cells (MSCs) in a subgroup of COVID-19 patients with acute respiratory distress has created potential benefits as supportive therapy for this viral contagion in patients with acute conditions and aged patients with severe pneumonia. Consequently, within this overview, we discuss the role and therapeutic potential of MSCs and the challenges ahead in using them to treat viral infections, with highlighting on COVID-19 infection.
Keywords: COVID-19; Mesenchymal stem cells; SARS-CoV-2; Stem cells therapy.
© 2022. The Author(s).
Conflict of interest statement
The authors have no relevant financial or non-financial interests to disclose.
Figures



Similar articles
-
Expanded Umbilical Cord Mesenchymal Stem Cells (UC-MSCs) as a Therapeutic Strategy in Managing Critically Ill COVID-19 Patients: The Case for Compassionate Use.Pain Physician. 2020 Mar;23(2):E71-E83. Pain Physician. 2020. PMID: 32214286
-
Menstrual Blood-Derived Mesenchymal Stem Cell Therapy for Severe COVID-19 Patients.Curr Stem Cell Res Ther. 2024;19(5):644-652. doi: 10.2174/1574888X18666230417085117. Curr Stem Cell Res Ther. 2024. PMID: 37073149 Review.
-
Clinical Consideration for Mesenchymal Stem Cells in Treatment of COVID-19.Curr Pharm Des. 2022;28(36):2991-2994. doi: 10.2174/1381612828666220926094730. Curr Pharm Des. 2022. PMID: 36165526
-
COVID-19 and its Therapeutics: Special Emphasis on Mesenchymal Stem Cells Based Therapy.Stem Cell Rev Rep. 2021 Feb;17(1):113-131. doi: 10.1007/s12015-020-10037-2. Stem Cell Rev Rep. 2021. PMID: 32920752 Free PMC article. Review.
-
Mesenchymal Stem Cell-Based Therapy for COVID-19: Possibility and Potential.Curr Stem Cell Res Ther. 2021;16(2):105-108. doi: 10.2174/1574888X15666200601152832. Curr Stem Cell Res Ther. 2021. PMID: 32479246 Review.
Cited by
-
Recent advances on high-efficiency of microRNAs in different types of lung cancer: a comprehensive review.Cancer Cell Int. 2023 Nov 20;23(1):284. doi: 10.1186/s12935-023-03133-z. Cancer Cell Int. 2023. PMID: 37986065 Free PMC article. Review.
-
Viruses and neurodegeneration: a growing concern.J Transl Med. 2025 Jan 12;23(1):46. doi: 10.1186/s12967-024-06025-6. J Transl Med. 2025. PMID: 39800721 Free PMC article. Review.
-
An overview on nanoparticle-based strategies to fight viral infections with a focus on COVID-19.J Nanobiotechnology. 2022 Oct 8;20(1):440. doi: 10.1186/s12951-022-01625-0. J Nanobiotechnology. 2022. PMID: 36209089 Free PMC article. Review.
-
Recent Advances in Crimean-Congo Hemorrhagic Fever Virus Detection, Treatment, and Vaccination: Overview of Current Status and Challenges.Biol Proced Online. 2024 Jun 26;26(1):20. doi: 10.1186/s12575-024-00244-3. Biol Proced Online. 2024. PMID: 38926669 Free PMC article. Review.
-
Recent advancements in nanomaterial-based biosensors for diagnosis of breast cancer: a comprehensive review.Cancer Cell Int. 2025 Feb 18;25(1):50. doi: 10.1186/s12935-025-03663-8. Cancer Cell Int. 2025. PMID: 39966938 Free PMC article. Review.
References
-
- Long Q-X, et al. Antibody responses to SARS-CoV-2 in patients with COVID-19. Nat Med. 2020;26:845–848. - PubMed
-
- Coronavirus N. Situation reports-World Health Organization (WHO). 2019.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

