Identification of storage conditions stabilizing extracellular vesicles preparations
- PMID: 35716060
- PMCID: PMC9206228
- DOI: 10.1002/jev2.12238
Identification of storage conditions stabilizing extracellular vesicles preparations
Abstract
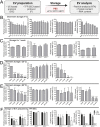
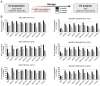
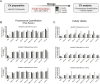
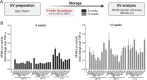
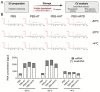
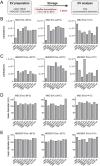
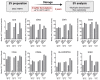
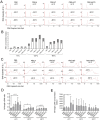
Extracellular vesicles (EVs) play a key role in many physiological and pathophysiological processes and hold great potential for therapeutic and diagnostic use. Despite significant advances within the last decade, the key issue of EV storage stability remains unresolved and under investigated. Here, we aimed to identify storage conditions stabilizing EVs and comprehensively compared the impact of various storage buffer formulations at different temperatures on EVs derived from different cellular sources for up to 2 years. EV features including concentration, diameter, surface protein profile and nucleic acid contents were assessed by complementary methods, and engineered EVs containing fluorophores or functionalized surface proteins were utilized to compare cellular uptake and ligand binding. We show that storing EVs in PBS over time leads to drastically reduced recovery particularly for pure EV samples at all temperatures tested, starting already within days. We further report that using PBS as diluent was found to result in severely reduced EV recovery rates already within minutes. Several of the tested new buffer conditions largely prevented the observed effects, the lead candidate being PBS supplemented with human albumin and trehalose (PBS-HAT). We report that PBS-HAT buffer facilitates clearly improved short-term and long-term EV preservation for samples stored at -80°C, stability throughout several freeze-thaw cycles, and drastically improved EV recovery when using a diluent for EV samples for downstream applications.
Keywords: diluent; exosomes; extracellular vesicles; liposomes; preservation; stability; storage; storage buffer; vesicles.
© 2022 The Authors. Journal of Extracellular Vesicles published by Wiley Periodicals, LLC on behalf of the International Society for Extracellular Vesicles.
Figures






References
-
- Barreiro, K. , Dwivedi, O. P. , Valkonen, S. , Groop, P. H. , Tuomi, T. , Holthofer, H. , Rannikko, A. , Yliperttula, M. , Siljander, P. , Laitinen, S. , Serkkola, E. , Af Hallstrom, T. , Forsblom, C. , Groop, L. , & Puhka, M. (2021). Urinary extracellular vesicles: Assessment of pre‐analytical variables and development of a quality control with focus on transcriptomic biomarker research. Journal of Extracellular Vesicles, 10(12), e12158. - PMC - PubMed
-
- Cavallaro, S. , Pevere, F. , Stridfeldt, F. , Gorgens, A. , Paba, C. , Sahu, S. S. , Mamand, D. R. , Gupta, D. , El Andaloussi, S. , Linnros, J. , & Dev, A. (2021). Multiparametric profiling of single nanoscale extracellular vesicles by combined atomic force and fluorescence microscopy: Correlation and heterogeneity in their molecular and biophysical features. Small, 17(14), e2008155. - PubMed
-
- Charoenviriyakul, C. , Takahashi, Y. , Nishikawa, M. , & Takakura, Y. (2018). Preservation of exosomes at room temperature using lyophilization. International Journal of Pharmaceutics, 553(1‐2), 1–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

