Cutaneous manifestations following COVID-19 vaccination: A report of 25 cases
- PMID: 35716105
- PMCID: PMC9349410
- DOI: 10.1111/dth.15651
Cutaneous manifestations following COVID-19 vaccination: A report of 25 cases
Abstract
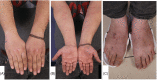
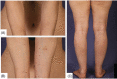
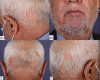
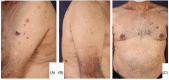
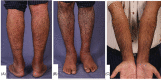
Various adverse effects particularly cutaneous manifestations associated with different COVID-19 vaccines have been observed in practice. The aim of our study was to evaluate all patients who presented to our tertiary center with skin manifestations following COVID-19 vaccines injection from September to December 2021. All patients with skin manifestation within 30 days or less following COVID-19 vaccination were enrolled in our case-series. All cases included in our study were diagnosed based on clinical and/or histopathological evaluation and all other possible differential diagnoses were ruled out. Twenty-five individuals including 16 (64%) males and 9 (36%) females with the mean age of 47 ± 17.62 years (range 18-91) were enrolled in our study. Twenty-two (88%) patients developed lesions after Sinopharm vaccine injection and 3 (12%) cases manifested lesions after the AstraZeneca vaccine. Six (24%) patients developed new-onset lichen planus (LP) and 1 (4%) patient manifested LP flare-up. Two (8%) individuals developed psoriasis and 1 (4%) case showed psoriasis exacerbation. One (4%) patient developed new-onset pemphigus vulgaris (PV) and 1 (4%) case experienced a flare of PV lesions. One (4%) patient manifested pityriasis lichenoides et varioliformis acuta (PLEVA) flare-up. Other new-onset cases were as follows: toxic epidermal necrolysis (TEN) (n = 1, 4%), bullous pemphigoid (BP) (n = 2, 8%), alopecia areata (AA) (n = 2, 8%), pytriasis rosea (n = 1, 4%), herpes zoster (n = 1, 4%), cutaneous small vessel vasculitis (n = 1, 4%), erythema multiform (EM) and urticaria (n = 3, 12%), and morphea (n = 1, 4%). Physicians should be aware of the possible side effects especially cutaneous manifestations associated with COVID-19 vaccines.
Keywords: COVID-19; COVID-19 vaccine; astrazeneca; side-effect; sinopharm.
© 2022 Wiley Periodicals LLC.
Figures
Comment in
-
New onset of palmoplantar keratosis triggered by COVID-19 vaccination.Dermatol Ther. 2022 Dec;35(12):e15937. doi: 10.1111/dth.15937. Epub 2022 Oct 19. Dermatol Ther. 2022. PMID: 36239020 Free PMC article. No abstract available.
Similar articles
-
Clinicopathological characteristics of cutaneous complications following COVID-19 vaccination: A case series.J Cosmet Dermatol. 2024 Mar;23(3):725-730. doi: 10.1111/jocd.16042. Epub 2023 Oct 29. J Cosmet Dermatol. 2024. PMID: 37899662
-
Abrupt onset of Sweet syndrome, pityriasis rubra pilaris, pityriasis lichenoides et varioliformis acuta and erythema multiforme: unravelling a possible common trigger, the COVID-19 vaccine.Clin Exp Dermatol. 2022 Feb;47(2):437-440. doi: 10.1111/ced.14970. Epub 2021 Oct 21. Clin Exp Dermatol. 2022. PMID: 34617317 Free PMC article. No abstract available.
-
Cutaneous manifestations of COVID-19 and COVID-19 vaccination.J Dermatol. 2023 Mar;50(3):280-289. doi: 10.1111/1346-8138.16651. Epub 2023 Jan 13. J Dermatol. 2023. PMID: 36636825
-
The Impact of COVID-19 Vaccination on Inflammatory Skin Disorders and Other Cutaneous Diseases: A Review of the Published Literature.Viruses. 2023 Jun 23;15(7):1423. doi: 10.3390/v15071423. Viruses. 2023. PMID: 37515110 Free PMC article. Review.
-
New Onset and Exacerbations of Psoriasis Following COVID-19 Vaccines: A Systematic Review.Am J Clin Dermatol. 2022 Nov;23(6):775-799. doi: 10.1007/s40257-022-00721-z. Epub 2022 Sep 1. Am J Clin Dermatol. 2022. PMID: 36048409 Free PMC article.
Cited by
-
Pityriasis Lichenoides Following SARS-CoV-2 Infection/Vaccination.Curr Dermatol Rep. 2023;12(1):27-32. doi: 10.1007/s13671-023-00380-1. Epub 2023 Jan 17. Curr Dermatol Rep. 2023. PMID: 36688177 Free PMC article. Review.
-
Onset of leukocytoclastic vasculitis following covid-19 vaccination: case based comprehensive review.Rheumatol Int. 2024 Nov;44(11):2621-2635. doi: 10.1007/s00296-024-05718-x. Epub 2024 Sep 16. Rheumatol Int. 2024. PMID: 39284920 Review.
-
Adverse Cutaneous Reactions Following COVID-19 Vaccination Among Patients with Pre-Existing Urticaria: A Cross-Sectional Study at a Tertiary Care Center in Saudi Arabia.Int J Gen Med. 2025 Jun 17;18:3227-3237. doi: 10.2147/IJGM.S520084. eCollection 2025. Int J Gen Med. 2025. PMID: 40547349 Free PMC article.
-
New Onset of Hair Loss Disorders During the Coronavirus Disease 2019 Pandemic: A Korean Nationwide Population-Based Study.Ann Dermatol. 2025 Aug;37(4):250-258. doi: 10.5021/ad.25.007. Ann Dermatol. 2025. PMID: 40736525 Free PMC article.
-
Cutaneous Reactions Following COVID-19 Vaccination: A Review of the Current Literature.Clin Cosmet Investig Dermatol. 2022 Nov 6;15:2369-2382. doi: 10.2147/CCID.S388245. eCollection 2022. Clin Cosmet Investig Dermatol. 2022. PMID: 36387962 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

