The Close Interaction of a C-F Bond with an Amide Carbonyl: Crystallographic and Spectroscopic Characterization
- PMID: 35716396
- PMCID: PMC9544880
- DOI: 10.1002/anie.202207966
The Close Interaction of a C-F Bond with an Amide Carbonyl: Crystallographic and Spectroscopic Characterization
Abstract
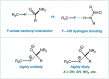
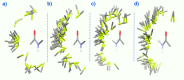
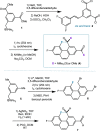
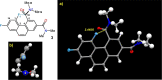
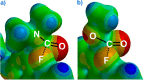
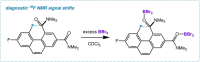
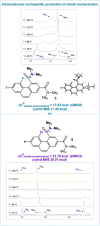
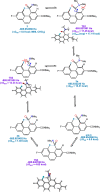
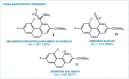
The putative interaction of a C-F bond with an amide carbonyl has been an intriguing topic of interest in this century for reasons spanning basic physical organic chemistry to biochemistry. However, to date, there exist no examples of a close, well-defined interaction in which its unique aspects can be identified and exploited. Herein, we finally present an engineered system possessing an exceptionally tight C-F-amide interaction, allowing us to obtain spectroscopic, crystallographic, and kinetic details of a distinctive, biochemically relevant chemical system for the first time. In turn, we also explore Lewis acid coordination, C-F bond promotion of amide isomerization, enantiomerization, and ion protonation processes.
Keywords: Amides; Fluorine; Non-Covalent Interactions.
© 2022 The Authors. Angewandte Chemie International Edition published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
-
- None
-
- Wermuth C., Molecular Variations Based on Isosteric Replacements. The Practice of Medicinal Chemistry, Elsevier, Amsterdam, 2002;
-
- O'Hagan D., Rzepa H., Chem. Commun. 1997, 645–652;
-
- Böhm H., Banner D., Bendels S., Kansy M., Kuhn B., Muller K., Obst-Sander U., Stahl M., ChemBioChem 2005, 5, 637–643; - PubMed
-
- Park B., Kitteringham N., Drug Metab. Rev. 1994, 26, 605–643. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

