Tanycytes control hypothalamic liraglutide uptake and its anti-obesity actions
- PMID: 35716660
- PMCID: PMC7613793
- DOI: 10.1016/j.cmet.2022.06.002
Tanycytes control hypothalamic liraglutide uptake and its anti-obesity actions
Abstract
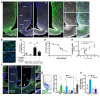
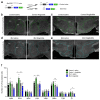
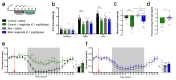
Liraglutide, an anti-diabetic drug and agonist of the glucagon-like peptide one receptor (GLP1R), has recently been approved to treat obesity in individuals with or without type 2 diabetes. Despite its extensive metabolic benefits, the mechanism and site of action of liraglutide remain unclear. Here, we demonstrate that liraglutide is shuttled to target cells in the mouse hypothalamus by specialized ependymoglial cells called tanycytes, bypassing the blood-brain barrier. Selectively silencing GLP1R in tanycytes or inhibiting tanycytic transcytosis by botulinum neurotoxin expression not only hampers liraglutide transport into the brain and its activation of target hypothalamic neurons, but also blocks its anti-obesity effects on food intake, body weight and fat mass, and fatty acid oxidation. Collectively, these striking data indicate that the liraglutide-induced activation of hypothalamic neurons and its downstream metabolic effects are mediated by its tanycytic transport into the mediobasal hypothalamus, strengthening the notion of tanycytes as key regulators of metabolic homeostasis.
Keywords: AAV; GLP1 analog; GLP1R agonist; arcuate nucleus of the hypothalamus; botulinum toxin; brain; fatty acid oxidation; median eminence; tanycyte; weight loss.
Copyright © 2022 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures
References
-
- Athauda D, Foltynie T. The glucagon-like peptide 1 (GLP) receptor as a therapeutic target in Parkinson’s disease: mechanisms of action. Drug Discovery Today. 2016;21:802–818. - PubMed
-
- Banks WA. The blood-brain barrier as an endocrine tissue. Nature reviews Endocrinology. 2019;15:444–455. - PubMed
-
- Beiroa D, Imbernon M, Gallego R, Senra A, Herranz D, Villarroya F, Serrano M, Fernø J, Salvador J, Escalada J, et al. GLP-1 agonism stimulates brown adipose tissue thermogenesis and browning through hypothalamic AMPK. Diabetes. 2014;63:3346–3358. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

