The contribution of hospital-acquired infections to the COVID-19 epidemic in England in the first half of 2020
- PMID: 35717168
- PMCID: PMC9206097
- DOI: 10.1186/s12879-022-07490-4
The contribution of hospital-acquired infections to the COVID-19 epidemic in England in the first half of 2020
Abstract
Background: SARS-CoV-2 is known to transmit in hospital settings, but the contribution of infections acquired in hospitals to the epidemic at a national scale is unknown.
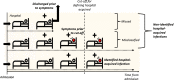
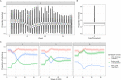
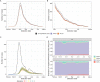
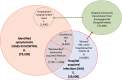
Methods: We used comprehensive national English datasets to determine the number of COVID-19 patients with identified hospital-acquired infections (with symptom onset > 7 days after admission and before discharge) in acute English hospitals up to August 2020. As patients may leave the hospital prior to detection of infection or have rapid symptom onset, we combined measures of the length of stay and the incubation period distribution to estimate how many hospital-acquired infections may have been missed. We used simulations to estimate the total number (identified and unidentified) of symptomatic hospital-acquired infections, as well as infections due to onward community transmission from missed hospital-acquired infections, to 31st July 2020.
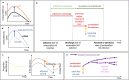
Results: In our dataset of hospitalised COVID-19 patients in acute English hospitals with a recorded symptom onset date (n = 65,028), 7% were classified as hospital-acquired. We estimated that only 30% (range across weeks and 200 simulations: 20-41%) of symptomatic hospital-acquired infections would be identified, with up to 15% (mean, 95% range over 200 simulations: 14.1-15.8%) of cases currently classified as community-acquired COVID-19 potentially linked to hospital transmission. We estimated that 26,600 (25,900 to 27,700) individuals acquired a symptomatic SARS-CoV-2 infection in an acute Trust in England before 31st July 2020, resulting in 15,900 (15,200-16,400) or 20.1% (19.2-20.7%) of all identified hospitalised COVID-19 cases.
Conclusions: Transmission of SARS-CoV-2 to hospitalised patients likely caused approximately a fifth of identified cases of hospitalised COVID-19 in the "first wave" in England, but less than 1% of all infections in England. Using time to symptom onset from admission for inpatients as a detection method likely misses a substantial proportion (> 60%) of hospital-acquired infections.
Keywords: COVID-19; Mathematical modelling; Nosocomial transmission; SARS-CoV-2.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Update of
-
The contribution of hospital-acquired infections to the COVID-19 epidemic in England in the first half of 2020.Res Sq [Preprint]. 2022 Mar 3:rs.3.rs-1140332. doi: 10.21203/rs.3.rs-1140332/v1. Res Sq. 2022. Update in: BMC Infect Dis. 2022 Jun 18;22(1):556. doi: 10.1186/s12879-022-07490-4. PMID: 35262072 Free PMC article. Updated. Preprint.
References
-
- Timeline: WHO’s COVID-19 response. 2021. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/interact.... Accessed 30 Mar 2021.
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

