Comprehensive characterization of the prostate tumor microenvironment identifies CXCR4/CXCL12 crosstalk as a novel antiangiogenic therapeutic target in prostate cancer
- PMID: 35717322
- PMCID: PMC9206324
- DOI: 10.1186/s12943-022-01597-7
Comprehensive characterization of the prostate tumor microenvironment identifies CXCR4/CXCL12 crosstalk as a novel antiangiogenic therapeutic target in prostate cancer
Abstract
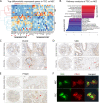
Background: Crosstalk between neoplastic and stromal cells fosters prostate cancer (PCa) progression and dissemination. Insight in cell-to-cell communication networks provides new therapeutic avenues to mold processes that contribute to PCa tumor microenvironment (TME) alterations. Here we performed a detailed characterization of PCa tumor endothelial cells (TEC) to delineate intercellular crosstalk between TEC and the PCa TME.
Methods: TEC isolated from 67 fresh radical prostatectomy (RP) specimens underwent multi-omic ex vivo characterization as well as orthogonal validation of both TEC functions and key markers by immunohistochemistry (IHC) and immunofluorescence (IF). To identify cell-cell interaction targets in TEC, we performed single-cell RNA sequencing (scRNA-seq) in four PCa patients who underwent a RP to catalogue cellular TME composition. Targets were cross-validated using IHC, publicly available datasets, cell culture expriments as well as a PCa xenograft mouse model.
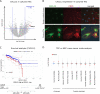
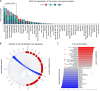
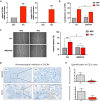
Results: Compared to adjacent normal endothelial cells (NEC) bulk RNA-seq analysis revealed upregulation of genes associated with tumor vasculature, collagen modification and extracellular matrix remodeling in TEC. PTGIR, PLAC9, CXCL12 and VDR were identified as TEC markers and confirmed by IF and IHC in an independent patient cohort. By scRNA-seq we identified 27 cell (sub)types, including endothelial cells (EC) with arterial, venous and immature signatures, as well as angiogenic tip EC. A focused molecular analysis revealed that arterial TEC displayed highest CXCL12 mRNA expression levels when compared to all other TME cell (sub)populations and showed a negative prognostic role. Receptor-ligand interaction analysis predicted interactions between arterial TEC derived CXCL12 and its cognate receptor CXCR4 on angiogenic tip EC. CXCL12 was in vitro and in vivo validated as actionable TEC target by highlighting the vessel number- and density- reducing activity of the CXCR4-inhibitor AMD3100 in murine PCa as well as by inhibition of TEC proliferation and migration in vitro.
Conclusions: Overall, our comprehensive analysis identified novel PCa TEC targets and highlights CXCR4/CXCL12 interaction as a potential novel target to interfere with tumor angiogenesis in PCa.
Keywords: Bulk RNA-seq; CXCR4/CXCL12; Prostate cancer; Single-cell RNA-seq; Target identification; Tip cell; Tumor endothelial cell.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

