Synergistic anti-SARS-CoV-2 activity of repurposed anti-parasitic drug combinations
- PMID: 35717393
- PMCID: PMC9206137
- DOI: 10.1186/s40360-022-00580-8
Synergistic anti-SARS-CoV-2 activity of repurposed anti-parasitic drug combinations
Abstract
Background: COVID-19 pandemic has claimed millions of lives and devastated the health service system, livelihood, and economy in many countries worldwide. Despite the vaccination programs in many countries, the spread of the pandemic continues, and effective treatment is still urgently needed. Although some antiviral drugs have been shown to be effective, they are not widely available. Repurposing of anti-parasitic drugs with in vitro anti-SARS-CoV-2 activity is a promising approach being tested in many clinical trials. Combination of these drugs is a plausible way to enhance their effectiveness.
Methods: The in vitro anti-SARS-CoV-2 activity of combinations of niclosamide, ivermectin and chloroquine were evaluated in Vero E6 and lung epithelial cells, Calu-3.
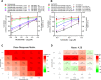
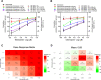
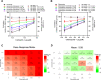
Results: All the two-drug combinations showed higher potency resulting in up to 4-fold reduction in the half maximal inhibitory concentration (IC50) values compared to individual drugs. Among these combinations, niclosamide-ivermectin achieved the highest inhibitory level of over 99%. Combination synergy analysis showed niclosamide-ivermectin combination to have the best synergy score with a mean Loewe synergy score of 4.28 and a peak synergy score of 24.6 in Vero E6 cells and a mean Loewe synergy score of 3.82 and a peak synergy score of 10.86 in Calu-3 cells.
Conclusions: The present study demonstrated the benefit of drug combinations on anti-SARS-CoV-2 activity. Niclosamide and ivermectin showed the best synergistic profile and should be further tested in clinical trials.
Keywords: Anti-parasitic drugs; Chloroquine; Ivermectin; Niclosamide; Repurposed drug; SARS-CoV-2.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



References
-
- FDA Approves First Treatment for COVID-19. https://www.fda.gov/news-events/press-announcements/fda-approves-first-t.... Accessed 15 Dec 2021.
-
- Coronavirus (COVID-19) Update: FDA Authorizes Drug Combination for Treatment of COVID-19. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19.... Accessed 1 Dec 2021.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

