Can't handle the stress? Mechanobiology and disease
- PMID: 35717527
- PMCID: PMC9420767
- DOI: 10.1016/j.molmed.2022.05.010
Can't handle the stress? Mechanobiology and disease
Abstract
Mechanobiology is a rapidly growing research area focused on how mechanical forces and properties influence biological systems at the cell, molecular, and tissue level, and how those biological systems, in turn, control mechanical parameters. Recently, it has become apparent that disrupted mechanobiology has a significant role in many diseases, from cardiovascular disease to muscular dystrophy and cancer. An improved understanding of this intricate process could be harnessed toward developing alternative and more targeted treatment strategies, and to advance the fields of regenerative and personalized medicine. Modulating the mechanical properties of the cellular microenvironment has already been used successfully to boost antitumor immune responses and to induce cardiac and spinal regeneration, providing inspiration for further research in this area.
Keywords: Mechanobiology; extracellular matrix; human disease; mechanotransduction; microenvironment; tissue mechanics.
Copyright © 2022 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of interests None declared by authors.
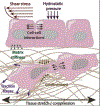
Figures


References
-
- Alonso JL and Goldmann WH, “Cellular mechanotransduction,” AIMS Biophysics, vol. 3, no. 1, 2016, doi: 10.3934/biophy.2016.1.50. - DOI
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

