The Staphylococcus aureus Network Adaptive Platform Trial Protocol: New Tools for an Old Foe
- PMID: 35717634
- PMCID: PMC9710697
- DOI: 10.1093/cid/ciac476
The Staphylococcus aureus Network Adaptive Platform Trial Protocol: New Tools for an Old Foe
Erratum in
-
Correction to: The Staphylococcus aureus Network Adaptive Platform Trial Protocol: New Tools for an Old Foe.Clin Infect Dis. 2023 Apr 17;76(8):1532-1533. doi: 10.1093/cid/ciac730. Clin Infect Dis. 2023. PMID: 36932686 Free PMC article. No abstract available.
Abstract
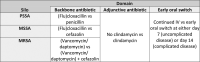
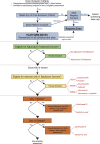
Staphylococcus aureus bloodstream (SAB) infection is a common and severe infectious disease, with a 90-day mortality of 15%-30%. Despite this, <3000 people have been randomized into clinical trials of treatments for SAB infection. The limited evidence base partly results from clinical trials for SAB infections being difficult to complete at scale using traditional clinical trial methods. Here we provide the rationale and framework for an adaptive platform trial applied to SAB infections. We detail the design features of the Staphylococcus aureus Network Adaptive Platform (SNAP) trial that will enable multiple questions to be answered as efficiently as possible. The SNAP trial commenced enrolling patients across multiple countries in 2022 with an estimated target sample size of 7000 participants. This approach may serve as an exemplar to increase efficiency of clinical trials for other infectious disease syndromes.
Keywords: Staphylococcus aureus; adaptive platform; bacteremia; bloodstream infection; randomized controlled trial.
© The Author(s) 2022. Published by Oxford University Press on behalf of Infectious Diseases Society of America.
Conflict of interest statement
Potential conflicts of interest. A. M. is an employee of Berry Consultants LLC, in which capacity she is contracted as a consultant to numerous pharmaceutical and device companies on topics of statistical modeling and trial design, and reports grants and contracts unrelated to this work and consulting fees from Berry Consultants LLC. A. C. B. reports participation as a member of the CAMERA-2 data and safety monitoring board (DSMB) and as chair of the Skin Trial DSMB; a role as Vice President of the World Society of Pediatric Infectious Diseases and as Co-Chair, Australian and New Zealand Paediatric Infectious Diseases group of the Australasian Society of Infectious Diseases (ASID). D. L. P. reports grants or contracts made to institution and unrelated to this work from Shionogi, Merck, and Pfizer; consulting fees to author from Antimicrobial Resistance Action Fund and Spero Therapeutics; payment or honoraria for lectures, presentations, speaker’s bureaus, manuscript writing, or educational events to author from Pfizer and Merck; support for attending meetings and/or travel to author from Pfizer; and an unpaid leadership or fiduciary role with ASID. J. A. R. reports contracts or grants paid to institution and unrelated to this work from the British Society of Antimicrobial Chemotherapy, bioMérieux, Pfizer, and QPEX; consulting fees paid to author from Gilead, Pfizer, Sandoz, Wolters Kluwer, Summit Pharma, and MSD; payment to author for lectures, presentations, speaker’s bureaus, manuscript writing, or educational events from MSD, Pfizer, and Cipla; an unpaid leadership or fiduciary role with Sepsis Clinical Care Standard, Australian Commission on Safety and Quality in Health Care, Consensus Guidelines on Prolonged Infusion of Beta-Lactam Antibiotics, American College of Clinical Pharmacy, Disease Modifying Treatment and Chemoprophylaxis, Australian National COVID-19 Clinical Evidence Taskforce, and Surviving Sepsis Guidelines. M. P. C. reports research support from the McGill Interdisciplinary Initiative in Infection and Immunity; research contracts from Cidara Therapeutics, Scynexis, and Amplyx; consulting fees as a scientific consultant for AstraZeneca; 3 pending patents (Methods for detecting tissue damage, graft-versus-host disease, and infections using cell-free DNA profiling; Methods for assessing the severity and progression of SARS-CoV-2 infections using cell-free DNA; and rapid identification of antimicrobial resistance and other microbial phenotypes using highly-multiplexed fluorescence in situ hybridization); stock options as a member of the scientific advisory board for GEn1E Lifesciences and Nomic Bio; and equity as co-founder of Kanvas Biosciences. R. J. L. is an employee of Berry Consultants, LLC, a statistical consulting firm that specializes in the design of adaptive and platform clinical trials. Berry Consultants received compensation for work included in the content of the submission. S. A. W. reports personal consulting fees from ClinicIQ pharma and Roche; an unpaid role as chair of Australian Clinical Trials Alliance; and stock and options with ClinicIQ. S. Y. C. T. reports a contract as a paid consultant for advice on clinical trial design, and consulting fees from Roivant Sciences as a paid consultant for advice on clinical trial design. T. C. L. reports research salary support from Fonds de Recherche Quebec–Sante, operating funds for other studies including CATCO from the CIHR; and operating funds for other studies from the McGill Interdisciplinary Institute Infection and Immunity. S. C. M. reports grants or contracts unrelated to this work from the HRC, and a nonremunerated role as the Chair of the New Zealand Microbiology Network. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Figures

References
-
- Coombs GW, Daley DA, Lee YT, Pang S; Australian Group on Antimicrobial Resistance . Australian Group on Antimicrobial Resistance (AGAR) Australian Staphylococcus aureus Sepsis Outcome Programme (ASSOP) annual report 2016. Commun Dis Intell 2018; 42:S2209-6051(18)00021-0. - PubMed

