Safety and immunogenicity of the inactivated whole-virus adjuvanted COVID-19 vaccine VLA2001: A randomized, dose escalation, double-blind phase 1/2 clinical trial in healthy adults
- PMID: 35718205
- PMCID: PMC9212764
- DOI: 10.1016/j.jinf.2022.06.009
Safety and immunogenicity of the inactivated whole-virus adjuvanted COVID-19 vaccine VLA2001: A randomized, dose escalation, double-blind phase 1/2 clinical trial in healthy adults
Abstract
Objectives: We aimed to evaluate the safety and optimal dose of a novel inactivated whole-virus adjuvanted vaccine against SARS-CoV-2: VLA2001.
Methods: We conducted an open-label, dose-escalation study followed by a double-blind randomized trial using low, medium and high doses of VLA2001 (1:1:1). The primary safety outcome was the frequency and severity of solicited local and systemic reactions within 7 days after vaccination. The primary immunogenicity outcome was the geometric mean titre (GMT) of neutralizing antibodies against SARS-CoV-2 two weeks after the second vaccination. The study is registered as NCT04671017.
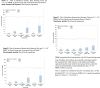
Results: Between December 16, 2020, and June 3, 2021, 153 healthy adults aged 18-55 years were recruited in the UK. Overall, 81.7% of the participants reported a solicited AE, with injection site tenderness (58.2%) and headache (46.4%) being the most frequent. Only 2 participants reported a severe solicited event. Up to day 106, 131 (85.6%) participants had reported any AE. All observed incidents were transient and non-life threatening in nature. Immunogenicity measured at 2 weeks after completion of the two-dose priming schedule, showed significantly higher GMTs of SARS-CoV-2 neutralizing antibody titres in the highest dose group (GMT 545.6; 95% CI: 428.1, 695.4) which were similar to a panel of convalescent sera (GMT 526.9; 95% CI: 336.5, 825.1). Seroconversion rates of neutralizing antibodies were also significantly higher in the high-dose group (>90%) compared to the other dose groups. In the high dose group, antigen-specific IFN-γ expressing T-cells reactive against the S, M and N proteins were observed in 76, 36 and 49%, respectively.
Conclusions: VLA2001 was well tolerated in all tested dose groups, and no safety signal of concern was identified. The highest dose group showed statistically significantly stronger immunogenicity with similar tolerability and safety, and was selected for phase 3 clinical development.
Keywords: Adjuvanted vaccine; COVID-19; Coronavirus; CpG 1018; Inactivated vaccine; Neutralizing antibody; RBD-binding IgG antibody; S protein binding IgG antibody; SARS-CoV-2; Whole-virus vaccine.
Copyright © 2022. Published by Elsevier Ltd.
Conflict of interest statement
Declaration of Competing Interest AF is a member of the Joint Committee on Vaccination and immunization and chair of the WHO European Technical Advisory Group of Experts on immunization. He is an investigator or provides consultative advice on clinical trials and studies of COVID-19 vaccines produced by AstraZeneca, Janssen, Valneva, Pfizer, and Sanofi, and of other vaccines from these and other manufacturers, including GlaxoSmithKline, VPI Pharmaceuticals, Takeda, and Bionet Asia. He receives no personal remuneration or benefits for any of this work. CTA, KD, SEL, RH, IC, BQ, and JCJ are employees of Valneva Austria GmbH.
Figures




References
-
- Mathieu E., Ritchie H., Ortiz-Ospina E., Roser M., Hasell J., Appel C., Giattino C., Rodés-Guirao L. A global database of COVID-19 vaccinations. Nat Hum Behav. 2021;5(7):947–953. doi: 10.1038/s41562-021-01122-8. . Epub 2021 May 10. Erratum in: Nat Hum Behav. 2021 Jun 17: PMID: 33972767. - DOI - PubMed
-
- World Health Organization . World Health Organization; 2021. Background document on the inactivated vaccine Sinovac-CoronaVac against COVID-19; pp. 1–30.
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

