Immunizing the imperfect immune system: Coronavirus disease 2019 vaccination in patients with inborn errors of immunity
- PMID: 35718282
- PMCID: PMC9212748
- DOI: 10.1016/j.anai.2022.06.009
Immunizing the imperfect immune system: Coronavirus disease 2019 vaccination in patients with inborn errors of immunity
Abstract
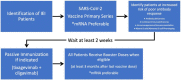
Objective: To update clinicians on current evidence regarding the immunogenicity and safety of coronavirus disease 2019 (COVID-19) vaccines in patients with inborn errors of immunity (IEI).
Data sources: Peer-reviewed, published studies in PubMed, clinical trials listed on ClinicalTrials.gov, and professional organization and governmental guidelines.
Study selections: Literature searches on PubMed and ClinicalTrials.gov were performed using a combination of the following keywords: primary immunodeficiency, COVID-19, SARS-CoV-2, and vaccination.
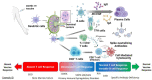
Results: A total of 26 studies met the criteria and were included in this review. Overall, antibody responses to COVID-19 vaccination were found in 72% of study subjects, with stronger responses observed after messenger RNA vaccination. Neutralizing antibodies were detected in patients with IEI, though consistently at lower levels than healthy controls. Risk factors for poor antibody responses included diagnosis of common variable immunodeficiency, presence of autoimmune comorbidities, and use of rituximab. T cell responses were detectable in most patients with IEI, with poorer responses often found in patients with common variable immunodeficiency. Safety of COVID-19 vaccines in patients with IEI was acceptable with high rates of reactogenicity but very few serious adverse events, including in patients with immune dysregulation.
Conclusion: COVID-19 vaccines are safe in patients with IEI and seem to be immunogenic in most individuals, with stronger responses found after messenger RNA vaccinations.
Copyright © 2022 American College of Allergy, Asthma & Immunology. All rights reserved.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

