In vitro study on efficacy of PHELA, an African traditional drug against SARS-CoV-2
- PMID: 35718800
- PMCID: PMC9207029
- DOI: 10.1038/s41598-022-13599-y
In vitro study on efficacy of PHELA, an African traditional drug against SARS-CoV-2
Abstract
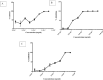
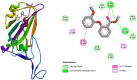
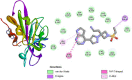
In 2019, coronavirus has made the third apparition in the form of SARS-CoV-2, a novel strain of coronavirus that is extremely pathogenic and it uses the same receptor as SARS-CoV, the angiotensin-converting enzyme 2 (ACE2). However, more than 182 vaccine candidates have been announced; and 12 vaccines have been approved for use, although, even vaccinated individuals are still vulnerable to infection. In this study, we investigated PHELA, recognized as an herbal combination of four exotic African medicinal plants namely; Clerodendrum glabrum E. Mey. Lamiaceae, Gladiolus dalenii van Geel, Rotheca myricoides (Hochst.) Steane & Mabb, and Senna occidentalis (L.) Link; as a candidate therapy for COVID-19. In vitro testing found that PHELA inhibited > 90% of SARS-CoV-2 and SARS-CoV infection at concentration levels of 0.005 mg/ml to 0.03 mg/ml and close to 100% of MERS-CoV infection at 0.1 mg/ml to 0.6 mg/ml. The in vitro average IC50 of PHELA on SARS-COV-2, SARS-CoV and MERS-COV were ~ 0.01 mg/ml. Secondly in silico docking studies of compounds identified in PHELA showed very strong binding energy interactions with the SARS-COV-2 proteins. Compound 5 showed the highest affinity for SARS-COV-2 protein compared to other compounds with the binding energy of - 6.8 kcal mol-1. Our data showed that PHELA has potential and could be developed as a COVID-19 therapeutic.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

