Gastrointestinal Changes and Alzheimer's Disease
- PMID: 35718965
- PMCID: PMC10497313
- DOI: 10.2174/1567205019666220617121255
Gastrointestinal Changes and Alzheimer's Disease
Abstract
Background: There is a well-described mechanism of communication between the brain and gastrointestinal system in which both organs influence the function of the other. This bi-directional communication suggests that disease in either organ may affect function in the other.
Objective: To assess whether the evidence supports gastrointestinal system inflammatory or degenerative pathophysiology as a characteristic of Alzheimer's disease (AD).
Methods: A review of both rodent and human studies implicating gastrointestinal changes in AD was performed.
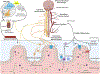
Results: Numerous studies indicate that AD changes are not unique to the brain but also occur at various levels of the gastrointestinal tract involving both immune and neuronal changes. In addition, it appears that numerous conditions and diseases affecting regions of the tract may communicate to the brain to influence disease.
Conclusion: Gastrointestinal changes represent an overlooked aspect of AD, representing a more system influence of this disease.
Keywords: Alzheimer; Microbiome; amyloid; enteric neuron; inflammation; intestine.
Copyright© Bentham Science Publishers; For any queries, please email at epub@benthamscience.net.
Conflict of interest statement
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Figures
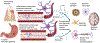

References
-
- Aziz Q, Thompson DG. Brain-gut axis in health and disease. Gastroenterology. 1998;114(3):559–78. - PubMed
-
- Waxenbaum JA, Varacallo M. Anatomy, autonomic nervous system. StatPearls [Internet]: StatPearls Publishing; 2019. - PubMed
-
- Furness JB, Callaghan BP, Rivera LR, Cho HJ. The enteric nervous system and gastrointestinal innervation: integrated local and central control. Advances in experimental medicine and biology. 2014;817:39–71. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

