Pyrimidoquinazolinophenanthroline Opens Next Chapter in Design of Bridging Ligands for Artificial Photosynthesis
- PMID: 35719124
- PMCID: PMC9546224
- DOI: 10.1002/chem.202200766
Pyrimidoquinazolinophenanthroline Opens Next Chapter in Design of Bridging Ligands for Artificial Photosynthesis
Abstract
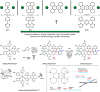
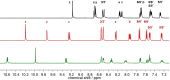
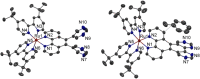
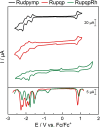
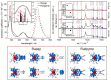
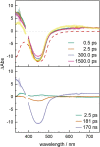
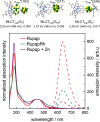
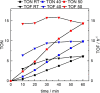
The synthesis and detailed characterization of a new Ru polypyridine complex containing a heteroditopic bridging ligand with previously unexplored metal-metal distances is presented. Due to the twisted geometry of the novel ligand, the resultant division of the ligand in two distinct subunits leads to steady state as well as excited state properties of the corresponding mononuclear Ru(II) polypyridine complex resembling those of prototype [Ru(bpy)3 ]2+ (bpy=2,2'-bipyridine). The localization of the initially optically excited and the nature of the long-lived excited states on the Ru-facing ligand spheres is evaluated by resonance Raman and fs-TA spectroscopy, respectively, and supported by DFT and TDDFT calculations. Coordination of a second metal (Zn or Rh) to the available bis-pyrimidyl-like coordination sphere strongly influences the frontier orbitals, apparent by, for example, luminescence quenching. Thus, the new bridging ligand motif offers electronic properties, which can be adjusted by the nature of the second metal center. Using the heterodinuclear Ru-Rh complex, visible light-driven reduction of NAD+ to NADH was achieved, highlighting the potential of this system for photocatalytic applications.
Keywords: artificial photosynthesis; bridging ligand; light-induced redox catalysis; quantum chemistry; ruthenium photosensitizer.
© 2022 The Authors. Chemistry - A European Journal published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Fischer W. W., Hemp J., Johnson J. E., Annu. Rev. Earth Planet. Sci. 2016, 44, 647–683.
-
- Harrison D. F., Weissberger E., Taube H., Science 1968, 159, 320–322. - PubMed
-
- Creutz C., Taube H., J. Am. Chem. Soc. 1969, 91, 3988–3989.
-
- Creutz C., Taube H., J. Am. Chem. Soc. 1973, 95, 1086–1094.
-
- Balzani V., Bergamini G., Marchioni F., Ceroni P., Coord. Chem. Rev. 2006, 250, 1254–1266.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

