Efficacy of topical lidocaine-prilocaine (EMLA®) for management of infant pain during pneumococcal vaccination: A randomized controlled trial
- PMID: 35719216
- PMCID: PMC9189906
- DOI: 10.1002/pne2.12070
Efficacy of topical lidocaine-prilocaine (EMLA®) for management of infant pain during pneumococcal vaccination: A randomized controlled trial
Abstract
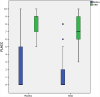
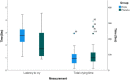
Few studies have evaluated whether topical anesthetic cream reduces pain during pneumococcal vaccination. This is crucial, since effective pain management should be evidence-based. Previous studies have shown that topical lidocaine-prilocaine (EMLA®) reduces vaccination-related pain, measured using pain-rating instruments and observation of crying time. This intervention study aimed to compare the efficacy of topical lidocaine-prilocaine cream with that of the standard of care on the expression of pain during the first pneumococcal vaccination administered at age 3 months under the Swedish national vaccination program. A randomized controlled trial included 72 infants receiving their first pneumococcal vaccination (Prevenar 13®). The study showed that topical lidocaine-prilocaine before pneumococcal vaccination significantly reduced infants' expression of pain according to the Face, Legs, Activity, Cry, Consolability (FLACC) score (P = .006) and increased latency to cry (P = .001). There were no statistically significant differences in the total crying time (P = .146) between the groups. Topical lidocaine-prilocaine cream reduced pain expression and increased latency to cry in infants receiving their first pneumococcal vaccine. Systematic efforts are needed to successfully implement the use of topical anesthetic cream and other effective non-pharmacological pain-relieving strategies during infant vaccination procedures.
Keywords: infants; randomized controlled study; topical anesthetic; vaccine‐related pain.
© 2021 The Authors. Paediatric and Neonatal Pain published by John Wiley & Sons Ltd.
Figures
Similar articles
-
Local Lidocaine-Prilocaine for Immunisation in Infants.Vaccines (Basel). 2024 Nov 27;12(12):1329. doi: 10.3390/vaccines12121329. Vaccines (Basel). 2024. PMID: 39771991 Free PMC article. Review.
-
A systematic review of lidocaine-prilocaine cream (EMLA) in the treatment of acute pain in neonates.Pediatrics. 1998 Feb;101(2):E1. doi: 10.1542/peds.101.2.e1. Pediatrics. 1998. PMID: 9445511
-
Randomized controlled trial of topical EMLA and breastfeeding for reducing pain during wDPT vaccination.Eur J Pediatr. 2013 Nov;172(11):1527-33. doi: 10.1007/s00431-013-2076-6. Epub 2013 Jun 29. Eur J Pediatr. 2013. PMID: 23812513 Clinical Trial.
-
Use of lidocaine-prilocaine cream for vaccination pain in infants.J Pediatr. 1994 Apr;124(4):643-8. doi: 10.1016/s0022-3476(05)83150-6. J Pediatr. 1994. PMID: 8151485 Clinical Trial.
-
Randomized controlled trial of topical EMLA and vapocoolant spray for reducing pain during wDPT vaccination.World J Pediatr. 2017 Jun;13(3):236-241. doi: 10.1007/s12519-017-0004-y. Epub 2017 Jan 19. World J Pediatr. 2017. PMID: 28101779 Clinical Trial.
Cited by
-
Local Lidocaine-Prilocaine for Immunisation in Infants.Vaccines (Basel). 2024 Nov 27;12(12):1329. doi: 10.3390/vaccines12121329. Vaccines (Basel). 2024. PMID: 39771991 Free PMC article. Review.
-
Efficacy of Lidocaine Topical Solution in Reducing Discomfort Reaction of Horses to Intramuscular Vaccination.Animals (Basel). 2022 Jun 28;12(13):1659. doi: 10.3390/ani12131659. Animals (Basel). 2022. PMID: 35804558 Free PMC article.
References
-
- Murat I, Gall O, Tourniaire B. Procedural pain in children: evidence‐based best practice and guidelines. Reg Anesth Pain Med. 2003;28(6):561‐572. - PubMed
-
- Abdel Razek A, AZ El‐Dein N. Effect of breast‐feeding on pain relief during infant immunization injections. Int J Nurs Pract. 2009;15(2):99‐104. - PubMed
-
- von Baeyer CL, Marche TA, Rocha EM, Salmon K. Children's memory for pain: overview and implications for practice. J Pain. 2004;5(5):241‐249. - PubMed
-
- Halperin SA, McGrath P, Smith B, Houston T. Lidocaine‐prilocaine patch decreases the pain associated with the subcutaneous administration of measles‐mumps‐rubella vaccine but does not adversely affect the antibody response. J Pediatr. 2000;136(6):789‐794. - PubMed
LinkOut - more resources
Full Text Sources
