Iron Deprivation Modulates the Exoproteome in Paracoccidioides brasiliensis
- PMID: 35719340
- PMCID: PMC9205457
- DOI: 10.3389/fcimb.2022.903070
Iron Deprivation Modulates the Exoproteome in Paracoccidioides brasiliensis
Abstract
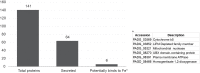
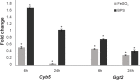
Fungi of the Paracoccidioides genus are the etiological agents of the systemic mycosis paracoccidioidomycosis and, when in the host, they find a challenging environment that is scarce in nutrients and micronutrients, such as Fe, which is indispensable for the survival of the pathogen. Previous studies have shown that fungi of this genus, in response to Fe deprivation, are able to synthesize and capture siderophores (Fe3+ chelators), use Fe-containing host proteins as a source of the metal, and use a non-canonical reductive pathway for Fe3+ assimilation. Despite all of these findings, there are still gaps that need to be filled in the pathogen response to metal deprivation. To contribute to the knowledge related to this subject, we obtained the exoproteome of Paracoccidioides brasiliensis (Pb18) undergoing Fe deprivation and by nanoUPLC-MSE. One hundred forty-one proteins were identified, and out of these, 64 proteins were predicted to be secreted. We also identified the regulation of several virulence factors. Among the results, we highlight Cyb5 as a secreted molecule of Paracoccidioides in the exoproteome obtained during Fe deprivation. Cyb5 is described as necessary for the Fe deprivation response of Saccharomyces cerevisiae and Aspergillus fumigatus. Experimental data and molecular modeling indicated that Cyb5 can bind to Fe ions in vitro, suggesting that it can be relevant in the arsenal of molecules related to iron homeostasis in P. brasiliensis.
Keywords: Fe; cytochrome b5 (CYB5); microbial adaptation; nutritional immunity; secretome.
Copyright © 2022 Souza, Pigosso, Silva, Galo, Paccez, e Silva, de Oliveira, Pereira and Soares.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Molecular Interactions of the Copper Chaperone Atx1 of Paracoccidioides brasiliensis with Fungal Proteins Suggest a Crosstalk between Iron and Copper Homeostasis.Microorganisms. 2023 Jan 18;11(2):248. doi: 10.3390/microorganisms11020248. Microorganisms. 2023. PMID: 36838213 Free PMC article.
-
MiRNAs regulate iron homeostasis in Paracoccidioides brasiliensis.Microbes Infect. 2021 Mar-Apr;23(2-3):104772. doi: 10.1016/j.micinf.2020.10.008. Epub 2020 Nov 4. Microbes Infect. 2021. PMID: 33157279
-
The "Little Iron Waltz": The Ternary Response of Paracoccidioides spp. to Iron Deprivation.J Fungi (Basel). 2020 Oct 12;6(4):221. doi: 10.3390/jof6040221. J Fungi (Basel). 2020. PMID: 33053811 Free PMC article. Review.
-
Distinct patterns of yeast cell morphology and host responses induced by representative strains of Paracoccidioides brasiliensis (Pb18) and Paracoccidioides lutzii (Pb01).Med Mycol. 2016 Feb;54(2):177-88. doi: 10.1093/mmy/myv072. Epub 2015 Sep 17. Med Mycol. 2016. PMID: 26384386
-
Paracoccidioides-host Interaction: An Overview on Recent Advances in the Paracoccidioidomycosis.Front Microbiol. 2015 Nov 25;6:1319. doi: 10.3389/fmicb.2015.01319. eCollection 2015. Front Microbiol. 2015. PMID: 26635779 Free PMC article. Review.
Cited by
-
Molecular Interactions of the Copper Chaperone Atx1 of Paracoccidioides brasiliensis with Fungal Proteins Suggest a Crosstalk between Iron and Copper Homeostasis.Microorganisms. 2023 Jan 18;11(2):248. doi: 10.3390/microorganisms11020248. Microorganisms. 2023. PMID: 36838213 Free PMC article.
References
-
- Bailão E. F. L. C., Parente A. F. A., Parente J. A., Silva-Bailão M. G., de Castro K.P, Kmetzsch L., et al. . (2012). Metal Acquisition and Homeostasis in Fungi. Curr. Fungal Infect. Rep. 6, 257–266. doi: 10.1007/s12281-012-0108-8 - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

