Post-COVID-19 Cholestasis: A Case Series and Review of Literature
- PMID: 35719861
- PMCID: PMC9187855
- DOI: 10.1016/j.jceh.2022.06.004
Post-COVID-19 Cholestasis: A Case Series and Review of Literature
Abstract
Background: Coronavirus disease-2019 (COVID-19) cholangiopathy is a recently known entity. There are very few reports of liver transplantation (LT) for COVID-19-induced cholangiopathy. It is well known that vaccines can prevent severe disease and improve outcomes. However, there are no reports on the impact of COVID-19 vaccines on cholestasis. Therefore, we aimed to compare the course and outcome of patients who developed cholestasis following COVID-19 infection among vaccinated and unvaccinated individuals. Methods: Patients diagnosed with post-COVID cholestasis during the pandemic were included in the study after excluding other causes of cholestasis.
Results: Eight unvaccinated and seven vaccinated individuals developed cholestasis following COVID-19 infection. Baseline demographics, presentation, severity, and management of COVID-19 were similar in both groups. However, patients in the unvaccinated group had a protracted course. The peak ALP was 312 (239-517) U/L in the vaccinated group and 571.5 (368-1058) U/L in the unvaccinated group (P = 0.02). Similarly, the peak γ-glutamyl transpeptidase values were lower in the vaccinated (325 [237-600] U/L) than in the unvaccinated group (832 [491-1640] U/L; P = 0.004). However, the peak values of total bilirubin, transaminases, and INR were similar in both groups. Five patients developed ascites gradually in the unvaccinated group whereas none in the vaccinated group developed ascites. Plasma exchange was done in five patients, and two were successfully bridged to living donor LT in the unvaccinated group. Only two patients recovered with conservative management in the unvaccinated group, whereas all recovered with conservative management in the vaccinated group. The other four patients in the unvaccinated group were planned for LT.
Conclusion: Post-COVID-19 cholestasis is associated with high morbidity and mortality, meriting early identification and appropriate management. Vaccination can modify the course of severe COVID-19 infection and improve outcomes.
Keywords: ALP, alkaline phosphatase; ALT, alanine transaminase; AST, aspartate transaminase; COVID-19, coronavirus disease-2019; DDLT, deceased donor living transplantation; GGT, γ-glutamyl transpeptidase; LDLT, living donor liver transplantation; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; UDCA, ursodeoxycholic acid; ULN, upper limit of normal; liver function test; liver transplantation; plasma exchange; vaccination.
© 2022 Indian National Association for Study of the Liver. Published by Elsevier B.V. All rights reserved.
Figures


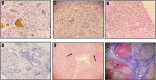
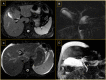
References
-
- Halpin S.J., McIvor C., Whyatt G., et al. Postdischarge symptoms and rehabilitation needs in survivors of COVID-19 infection: a cross-sectional evaluation. J Med Virol. 2021;93:1013–1022. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

