A Lack of Effectiveness in the ATM-Orchestrated DNA Damage Response Contributes to the DNA Repair Defect of HPV-Positive Head and Neck Cancer Cells
- PMID: 35719921
- PMCID: PMC9204973
- DOI: 10.3389/fonc.2022.765968
A Lack of Effectiveness in the ATM-Orchestrated DNA Damage Response Contributes to the DNA Repair Defect of HPV-Positive Head and Neck Cancer Cells
Abstract
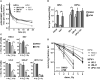
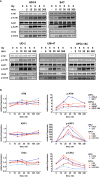
Patients with human papillomavirus-positive squamous cell carcinoma of the head and neck (HPV+ HNSCC) have a favorable prognosis compared to those with HPV-negative (HPV-) ones. We have shown previously that HPV+ HNSCC cell lines are characterized by enhanced radiation sensitivity and impaired DNA double-strand break (DSB) repair. Since then, various publications have suggested a defect in homologous recombination (HR) and dysregulated expression of DSB repair proteins as underlying mechanisms, but conclusions were often based on very few cell lines. When comparing the expression levels of suggested proteins and other key repair factors in 6 HPV+ vs. 5 HPV- HNSCC strains, we could not confirm most of the published differences. Furthermore, HPV+ HNSCC strains did not demonstrate enhanced sensitivity towards PARP inhibition, questioning a general HR defect. Interestingly, our expression screen revealed minimal levels of the central DNA damage response kinase ATM in the two most radiosensitive HPV+ strains. We therefore tested whether insufficient ATM activity may contribute to the enhanced cellular radiosensitivity. Irrespective of their ATM expression level, radiosensitive HPV+ HNSCC cells displayed DSB repair kinetics similar to ATM-deficient cells. Upon ATM inhibition, HPV+ cell lines showed only a marginal increase in residual radiation-induced γH2AX foci and induction of G2 cell cycle arrest as compared to HPV- ones. In line with these observations, ATM inhibition sensitized HPV+ HNSCC strains less towards radiation than HPV- strains, resulting in similar levels of sensitivity. Unexpectedly, assessment of the phosphorylation kinetics of the ATM targets KAP-1 and Chk2 as well as ATM autophosphorylation after radiation did not indicate directly compromised ATM activity in HPV-positive cells. Furthermore, ATM inhibition delayed radiation induced DNA end resection in both HPV+ and HPV- cells to a similar extent, further suggesting comparable functionality. In conclusion, DNA repair kinetics and a reduced effectiveness of ATM inhibition clearly point to an impaired ATM-orchestrated DNA damage response in HPV+ HNSCC cells, but since ATM itself is apparently functional, the molecular mechanisms need to be further explored.
Keywords: DNA damage response (DDR); DNA double-strand break repair; ataxia telangiectasia mutated (ATM); head and neck cancer; human papillomavirus (HPV); radiation sensitivity.
Copyright © 2022 Köcher, Zech, Krug, Gatzemeier, Christiansen, Meyer, Rietow, Struve, Mansour, Kriegs, Petersen, Betz, Rothkamm and Rieckmann.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




Similar articles
-
Tissue microarray analyses of the essential DNA repair factors ATM, DNA-PKcs and Ku80 in head and neck squamous cell carcinoma.Radiat Oncol. 2024 Oct 30;19(1):150. doi: 10.1186/s13014-024-02541-3. Radiat Oncol. 2024. PMID: 39478631 Free PMC article.
-
Patient derived ex vivo tissue slice cultures demonstrate a profound DNA double-strand break repair defect in HPV-positive oropharyngeal head and neck cancer.Radiother Oncol. 2022 Mar;168:138-146. doi: 10.1016/j.radonc.2022.01.017. Epub 2022 Jan 29. Radiother Oncol. 2022. PMID: 35093407
-
Targeting DNA Double-Strand Break Repair Enhances Radiosensitivity of HPV-Positive and HPV-Negative Head and Neck Squamous Cell Carcinoma to Photons and Protons.Cancers (Basel). 2020 Jun 7;12(6):1490. doi: 10.3390/cancers12061490. Cancers (Basel). 2020. PMID: 32517381 Free PMC article.
-
The radiobiology of HPV-positive and HPV-negative head and neck squamous cell carcinoma.Expert Rev Mol Med. 2020 Jul 2;22:e3. doi: 10.1017/erm.2020.4. Expert Rev Mol Med. 2020. PMID: 32611474 Free PMC article. Review.
-
The influence of heterochromatin on DNA double strand break repair: Getting the strong, silent type to relax.DNA Repair (Amst). 2010 Dec 10;9(12):1273-82. doi: 10.1016/j.dnarep.2010.09.013. Epub 2010 Oct 30. DNA Repair (Amst). 2010. PMID: 21036673 Review.
Cited by
-
Tissue microarray analyses of the essential DNA repair factors ATM, DNA-PKcs and Ku80 in head and neck squamous cell carcinoma.Radiat Oncol. 2024 Oct 30;19(1):150. doi: 10.1186/s13014-024-02541-3. Radiat Oncol. 2024. PMID: 39478631 Free PMC article.
-
Association of oropharyngeal cancer recurrence with tumor-intrinsic and immune-mediated sequelae of reduced genomic instability.bioRxiv [Preprint]. 2024 Nov 4:2024.10.31.621311. doi: 10.1101/2024.10.31.621311. bioRxiv. 2024. PMID: 39574723 Free PMC article. Preprint.
-
DNA Damage Response Mechanisms in Head and Neck Cancer: Significant Implications for Therapy and Survival.Int J Mol Sci. 2023 Feb 1;24(3):2760. doi: 10.3390/ijms24032760. Int J Mol Sci. 2023. PMID: 36769087 Free PMC article. Review.
-
HPV-Mediated Radiosensitivity in Oropharyngeal Squamous Cell Carcinoma: Molecular Mechanisms and Cellular Pathways.Curr Oncol Rep. 2025 May;27(5):634-641. doi: 10.1007/s11912-025-01666-2. Epub 2025 Apr 11. Curr Oncol Rep. 2025. PMID: 40214894 Free PMC article. Review.
-
The Causes and Consequences of DNA Damage and Chromosomal Instability Induced by Human Papillomavirus.Cancers (Basel). 2024 Apr 25;16(9):1662. doi: 10.3390/cancers16091662. Cancers (Basel). 2024. PMID: 38730612 Free PMC article. Review.
References
-
- Lohaus F, Linge A, Tinhofer I, Budach V, Gkika E, Stuschke M, et al. . HPV16 DNA Status Is a Strong Prognosticator of Loco-Regional Control After Postoperative Radiochemotherapy of Locally Advanced Oropharyngeal Carcinoma: Results From a Multicentre Explorative Study of the German Cancer Consortium Radiation Oncology Group (DKTK-ROG). Radiother Oncol (2014) 113(3):317–23. doi: 10.1016/j.radonc.2014.11.011 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

