Endoscopic Endonasal Supraoptic and Infraoptic Approaches for Complex "Parasuprasellar" Lesions: Surgical Anatomy, Technique Nuances, and Case Series
- PMID: 35719989
- PMCID: PMC9204328
- DOI: 10.3389/fonc.2022.847250
Endoscopic Endonasal Supraoptic and Infraoptic Approaches for Complex "Parasuprasellar" Lesions: Surgical Anatomy, Technique Nuances, and Case Series
Abstract
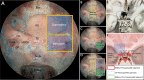
Objective: The surgical management of lesions involving the lateral area of the suprasellar region, including the lateral aspect of the planum sphenoidale and the tight junction region of the optic canal (OC), anterior clinoid process (ACP), and internal carotid artery (ICA) and its dural rings, is extremely challenging. Here, these regions, namely, the "parasuprasellar" area, are described from the endonasal perspective. Moreover, the authors introduce two novels endoscopic endonasal supraoptic (EESO) and endoscopic endonasal infraoptic (EEIO) approaches to access the parasuprasellar area.
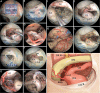
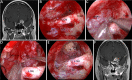
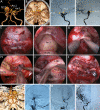
Methods: Surgical simulation of the EESO and EEIO approaches to the parasuprasellar area was conducted in 5 silicon-injected specimens. The same techniques were applied in 12 patients with lesions involving the parasuprasellar area.
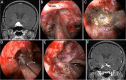
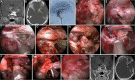
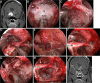
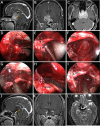
Results: The EESO approach provided excellent surgical access to the lateral region of the planum sphenoidale, which corresponds to the orbital gyrus of the frontal lobe. With stepwise bone (OC, optic strut and ACP) removal, dissociation of the ophthalmic artery (OA) and optic nerve (ON), the EEIO approach enables access to the lateral region of the supraclinoidal ICA. These approaches can be used independently or in combination, but are more often employed as a complement to the endoscopic endonasal midline and transcavernous approaches. In clinical application, the EESO and EEIO approaches were successfully performed in 12 patients harboring tumors as well as multiple aneurysms involving the parasuprasellar area. Gross total and subtotal tumor resection were achieved in 9 patients and 1 patient, respectively. For two patients with multiple aneurysms, the lesions were clipped selectively according to location and size. Visual acuity improved in 7 patients, remained stable in 4, and deteriorated in only 1. No postoperative intracranial infection or ICA injury occurred in this series.
Conclusions: The EESO and EEIO approaches offer original treatment options for well-selected lesions involving the parasuprasellar area. They can be combined with the endoscopic endonasal midline and transcavernous approaches to remove extensive pathologies involving the intrasellar, suprasellar, sphenoid, and cavernous sinuses and even the bifurcation of the ICA. This work for the first time pushes the boundary of the endoscopic endonasal approach lateral to the supraclinoidal ICA and ON.
Keywords: anterior clinoid process; endoscopic endonasal approach; internal carotid artery; optic canal; parasuprasellar area; surgical technique.
Copyright © 2022 Bao, Yang, Zhou, Xie, Wu, Ding, Wu, Xiao, Yang, Tang and Hong.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Lateral compartment of the cavernous sinus from the endoscopic endonasal approach: anatomical considerations and surgical relevance to adenoma surgery.J Neurosurg. 2024 Aug 9;142(2):475-487. doi: 10.3171/2024.4.JNS232662. Print 2025 Feb 1. J Neurosurg. 2024. PMID: 39126713
-
Endoscopic endonasal approach for a tuberculum sellae meningioma.Neurosurg Focus. 2012 Jan;32 Suppl 1:E8. doi: 10.3171/2012.V8.FOCUS11308. Neurosurg Focus. 2012. PMID: 26018978
-
Endoscopic endonasal anterior clinoidectomy: surgical anatomy, technique nuance, and case series.J Neurosurg. 2019 Jul 5;133(2):451-461. doi: 10.3171/2019.4.JNS183213. Print 2020 Aug 1. J Neurosurg. 2019. PMID: 31277066
-
Extended endoscopic endonasal approach to the midline skull base: the evolving role of transsphenoidal surgery.Adv Tech Stand Neurosurg. 2008;33:151-99. doi: 10.1007/978-3-211-72283-1_4. Adv Tech Stand Neurosurg. 2008. PMID: 18383814 Review.
-
Surgical Anatomy Applied to the Resection of Craniopharyngiomas: Anatomic Compartments and Surgical Classifications.World Neurosurg. 2020 Oct;142:611-625. doi: 10.1016/j.wneu.2020.05.171. World Neurosurg. 2020. PMID: 32987617 Review.
Cited by
-
Distinction of papillary and adamantinomatous craniopharyngioma: Clinical features, surgical nuances and hypothalamic outcomes.Neoplasia. 2024 Nov;57:101060. doi: 10.1016/j.neo.2024.101060. Epub 2024 Oct 1. Neoplasia. 2024. PMID: 39357265 Free PMC article.
-
Surgical treatment of a giant paraophthalmic aneurysm postfailed flow diversion through endoscopic endonasal approach: Technical nuances and review of the literature.Surg Neurol Int. 2024 Mar 8;15:75. doi: 10.25259/SNI_939_2023. eCollection 2024. Surg Neurol Int. 2024. PMID: 38628543 Free PMC article.
-
Operative management of trigeminal schwannomas: based on a modified classification in a study of 93 cases.Acta Neurochir (Wien). 2023 Dec;165(12):4157-4168. doi: 10.1007/s00701-023-05857-3. Epub 2023 Nov 24. Acta Neurochir (Wien). 2023. PMID: 37999914 Review.
References
LinkOut - more resources
Full Text Sources
Miscellaneous

