Effect of PARP Inhibitor Combined with Bevacizumab on Platinum-Resistant Recurrent Ovarian Epithelial Carcinoma
- PMID: 35720032
- PMCID: PMC9205723
- DOI: 10.1155/2022/4600145
Effect of PARP Inhibitor Combined with Bevacizumab on Platinum-Resistant Recurrent Ovarian Epithelial Carcinoma
Retraction in
-
Retracted: Effect of PARP Inhibitor Combined with Bevacizumab on Platinum-Resistant Recurrent Ovarian Epithelial Carcinoma.Comput Math Methods Med. 2023 Jun 28;2023:9823491. doi: 10.1155/2023/9823491. eCollection 2023. Comput Math Methods Med. 2023. PMID: 37416219 Free PMC article.
Abstract
Objective: This study was aimed at investigating the efficacy of PARP inhibitor combined with bevacizumab in the treatment of platinum-resistant recurrent ovarian epithelial carcinoma.
Methods: A total of 84 patients with platinum-resistant recurrent ovarian epithelial carcinoma treated in our hospital from May 2017 to June 2018 were selected as the research objects. The patients were divided into observation group (n = 42) and control group (n = 42) according to random number table method. The observation group was treated with olaparib combined with bevacizumab, while the control group was treated with albumin-bound paclitaxel combined with bevacizumab, and the clinical efficacy of the two groups was observed. The levels of serum carbohydrate antigen 125 (CA125), carbohydrate antigen 199 (CA199), and epididymal protein 4 (HE4) were determined. The levels of miRNA124, mirNA-21, and miRNA-203 in the two groups were detected. The incidence of adverse reactions was compared between the two groups. The quality of life of the two groups was assessed using FACT-G scale. The drug safety of the two groups was observed. All patients were followed up for 3 years, and the survival time of the two groups was recorded. The Kaplan-Meier method was used to analyze the survival of the two groups.
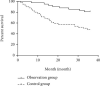
Results: The overall response rate (ORR) (69.05%) and disease control rate (DCR) (88.10%) of the observation group were higher than those of the control group (40.48% and 66.67%), and the differences were statistically significant (both P < 0.05). After treatment, the levels of serum CA125, CA199, HE4, miRNA124, miRNA-21, and miRNA-203 and the improvement degree of quality of life score in the observation group were greater than those in the control group, with statistical significances (all P < 0.05).The 1-year, 2-year, and 3-year survival rates of the observation group (97.62%, 88.10%, and 80.95%) were higher than those of the control group (71.43%, 57.14%, and 47.62%), with statistical significances (all P > 0.05).
Conclusion: PARP inhibitor combined with bevacizumab had good effect in the treatment of platinum-resistant recurrent ovarian epithelial carcinoma and can effectively improve the survival time and quality of life of patients.
Copyright © 2022 Li Sun et al.
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Biomarker-driven targeted therapy in patients with recurrent platinum-resistant epithelial ovarian cancer (BRIGHT): protocol for an open-label, multicenter, umbrella study.Int J Gynecol Cancer. 2024 Sep 2;34(9):1461-1465. doi: 10.1136/ijgc-2024-005351. Int J Gynecol Cancer. 2024. PMID: 38658024
-
Efficacy of Bevacizumab Combined with Albumin-Bound Paclitaxel in the Treatment of Platinum-Resistant Recurrent Ovarian Cancer.J BUON. 2019 Nov-Dec;24(6):2303-2309. J BUON. 2019. PMID: 31983098
-
Efficacy and Safety of Platinum-based Chemotherapy With Bevacizumab Followed by Bevacizumab Maintenance for Recurrent Ovarian, Fallopian Tube, and Primary Peritoneal Cancer During PARP Inhibitor Therapy: A Multicenter Retrospective Study.Anticancer Res. 2023 Mar;43(3):1265-1272. doi: 10.21873/anticanres.16273. Anticancer Res. 2023. PMID: 36854492
-
Rationale for combination PARP inhibitor and antiangiogenic treatment in advanced epithelial ovarian cancer: A review.Gynecol Oncol. 2021 Aug;162(2):482-495. doi: 10.1016/j.ygyno.2021.05.018. Epub 2021 Jun 3. Gynecol Oncol. 2021. PMID: 34090705 Review.
-
Prise en charge médicale de la récidive du cancer épithélial de l'ovaire: Medical management of recurrent epithelial ovarian cancer.Bull Cancer. 2021 Dec;108(9S1):S22-S32. doi: 10.1016/S0007-4551(21)00584-1. Bull Cancer. 2021. PMID: 34955159 Review.
Cited by
-
Retracted: Effect of PARP Inhibitor Combined with Bevacizumab on Platinum-Resistant Recurrent Ovarian Epithelial Carcinoma.Comput Math Methods Med. 2023 Jun 28;2023:9823491. doi: 10.1155/2023/9823491. eCollection 2023. Comput Math Methods Med. 2023. PMID: 37416219 Free PMC article.
-
Effect of bevacizumab plus paclitaxel and carboplatin regimen on prognostic survival of ovarian cancer patients.Am J Transl Res. 2022 Dec 15;14(12):8761-8767. eCollection 2022. Am J Transl Res. 2022. PMID: 36628241 Free PMC article.
-
Ovarian Cancer-Insights into Platinum Resistance and Overcoming It.Medicina (Kaunas). 2023 Mar 10;59(3):544. doi: 10.3390/medicina59030544. Medicina (Kaunas). 2023. PMID: 36984544 Free PMC article. Review.
-
Incidence and risk of hypertension associated with PARP inhibitors in cancer patients: a systematic review and meta-analysis.BMC Cancer. 2023 Jan 31;23(1):107. doi: 10.1186/s12885-023-10571-5. BMC Cancer. 2023. PMID: 36717798 Free PMC article.
References
-
- Lheureux S., Cristea M. C., Bruce J. P., et al. Adavosertib plus gemcitabine for platinum-resistant or platinum-refractory recurrent ovarian cancer: a double-blind, randomised, placebo-controlled, phase 2 trial. The Lancet . 2021;397(10271):281–292. doi: 10.1016/s0140-6736(20)32554-x. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous