The Effects of Carbon Source and Growth Temperature on the Fatty Acid Profiles of Thermobifida fusca
- PMID: 35720111
- PMCID: PMC9198275
- DOI: 10.3389/fmolb.2022.896226
The Effects of Carbon Source and Growth Temperature on the Fatty Acid Profiles of Thermobifida fusca
Abstract
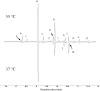
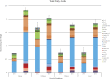
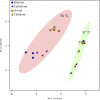
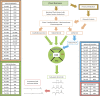
The aerobic, thermophilic Actinobacterium, Thermobifida fusca has been proposed as an organism to be used for the efficient conversion of plant biomass to fatty acid-derived precursors of biofuels or biorenewable chemicals. Despite the potential of T. fusca to catabolize plant biomass, there is remarkably little data available concerning the natural ability of this organism to produce fatty acids. Therefore, we determined the fatty acids that T. fusca produces when it is grown on different carbon sources (i.e., glucose, cellobiose, cellulose and avicel) and at two different growth temperatures, namely at the optimal growth temperature of 50°C and at a suboptimal temperature of 37°C. These analyses establish that T. fusca produces a combination of linear and branched chain fatty acids (BCFAs), including iso-, anteiso-, and 10-methyl BCFAs that range between 14- and 18-carbons in length. Although different carbon sources and growth temperatures both quantitatively and qualitatively affect the fatty acid profiles produced by T. fusca, growth temperature is the greater modifier of these traits. Additionally, genome scanning enabled the identification of many of the fatty acid biosynthetic genes encoded by T. fusca.
Keywords: Actinomycete; Thermobifida fusca; branched chain fatty acids; fatty acid biosynthesis pathway; fatty acid synthase; gas chromatography- mass spectrometry; principal component analysis.
Copyright © 2022 Winkelman and Nikolau.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Metabolic Profile of the Cellulolytic Industrial Actinomycete Thermobifida fusca.Metabolites. 2017 Nov 11;7(4):57. doi: 10.3390/metabo7040057. Metabolites. 2017. PMID: 29137138 Free PMC article.
-
Proteomics-based metabolic modeling and characterization of the cellulolytic bacterium Thermobifida fusca.BMC Syst Biol. 2014 Aug 13;8:86. doi: 10.1186/s12918-014-0086-2. BMC Syst Biol. 2014. PMID: 25115351 Free PMC article.
-
Driving carbon flux through exogenous butyryl-CoA: Acetate CoA-transferase to produce butyric acid at high titer in Thermobifida fusca.J Biotechnol. 2015 Dec 20;216:151-7. doi: 10.1016/j.jbiotec.2015.10.022. Epub 2015 Nov 1. J Biotechnol. 2015. PMID: 26535965
-
Biochemistry and genetics of actinomycete cellulases.Crit Rev Biotechnol. 1992;12(1-2):45-63. doi: 10.3109/07388559209069187. Crit Rev Biotechnol. 1992. PMID: 1733521 Review.
-
The cellulolytic system of Thermobifida fusca.Crit Rev Microbiol. 2014 Aug;40(3):236-47. doi: 10.3109/1040841X.2013.776512. Epub 2013 Mar 28. Crit Rev Microbiol. 2014. PMID: 23537325 Review.
Cited by
-
GC/MS Fatty Acid Profile of Marine-Derived Actinomycetes from Extreme Environments: Chemotaxonomic Insights and Biotechnological Potential.Mar Drugs. 2024 Dec 24;23(1):1. doi: 10.3390/md23010001. Mar Drugs. 2024. PMID: 39852503 Free PMC article.
References
LinkOut - more resources
Full Text Sources

