The Impact of Glycoengineering on the Endoplasmic Reticulum Quality Control System in Yeasts
- PMID: 35720120
- PMCID: PMC9201249
- DOI: 10.3389/fmolb.2022.910709
The Impact of Glycoengineering on the Endoplasmic Reticulum Quality Control System in Yeasts
Abstract
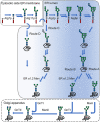
Yeasts are widely used and established production hosts for biopharmaceuticals. Despite of tremendous advances on creating human-type N-glycosylation, N-glycosylated biopharmaceuticals manufactured with yeasts are missing on the market. The N-linked glycans fulfill several purposes. They are essential for the properties of the final protein product for example modulating half-lives or interactions with cellular components. Still, while the protein is being formed in the endoplasmic reticulum, specific glycan intermediates play crucial roles in the folding of or disposal of proteins which failed to fold. Despite of this intricate interplay between glycan intermediates and the cellular machinery, many of the glycoengineering approaches are based on modifications of the N-glycan processing steps in the endoplasmic reticulum (ER). These N-glycans deviate from the canonical structures required for interactions with the lectins of the ER quality control system. In this review we provide a concise overview on the N-glycan biosynthesis, glycan-dependent protein folding and quality control systems and the wide array glycoengineering approaches. Furthermore, we discuss how the current glycoengineering approaches partially or fully by-pass glycan-dependent protein folding mechanisms or create structures that mimic the glycan epitope required for ER associated protein degradation.
Keywords: endoplasmic reticulum associated protein degradation (ERAD); endoplasmic reticulum quality control (ERQC); glycoengineering; protein N-glycosylation; yeast.
Copyright © 2022 Piirainen and Frey.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Investigating the role of ERAD on antibody processing in glycoengineered Saccharomyces cerevisiae.FEMS Yeast Res. 2020 Feb 1;20(1):foaa002. doi: 10.1093/femsyr/foaa002. FEMS Yeast Res. 2020. PMID: 31922547
-
N-glycosylation, a leading role in viral infection and immunity development.Mol Biol Rep. 2022 Aug;49(8):8109-8120. doi: 10.1007/s11033-022-07359-4. Epub 2022 Apr 1. Mol Biol Rep. 2022. PMID: 35364718 Free PMC article. Review.
-
The Crucial Role of Demannosylating Asparagine-Linked Glycans in ERADicating Misfolded Glycoproteins in the Endoplasmic Reticulum.Front Plant Sci. 2021 Jan 12;11:625033. doi: 10.3389/fpls.2020.625033. eCollection 2020. Front Plant Sci. 2021. PMID: 33510762 Free PMC article. Review.
-
The evolution of N-glycan-dependent endoplasmic reticulum quality control factors for glycoprotein folding and degradation.Proc Natl Acad Sci U S A. 2007 Jul 10;104(28):11676-81. doi: 10.1073/pnas.0704862104. Epub 2007 Jul 2. Proc Natl Acad Sci U S A. 2007. PMID: 17606910 Free PMC article.
-
Mannose trimming is required for delivery of a glycoprotein from EDEM1 to XTP3-B and to late endoplasmic reticulum-associated degradation steps.J Biol Chem. 2011 Jan 14;286(2):1292-300. doi: 10.1074/jbc.M110.154849. Epub 2010 Nov 9. J Biol Chem. 2011. PMID: 21062743 Free PMC article.
Cited by
-
Beyond In Vivo, Pharmaceutical Molecule Production in Cell-Free Systems and the Use of Noncanonical Amino Acids Therein.Chem Rev. 2025 Feb 12;125(3):1303-1331. doi: 10.1021/acs.chemrev.4c00126. Epub 2025 Jan 22. Chem Rev. 2025. PMID: 39841856 Free PMC article. Review.
-
Endoplasmic reticulum stress and quality control in relation to cisplatin resistance in tumor cells.Front Pharmacol. 2024 Jun 14;15:1419468. doi: 10.3389/fphar.2024.1419468. eCollection 2024. Front Pharmacol. 2024. PMID: 38948460 Free PMC article. Review.
References
Publication types
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

