Novel Therapeutic Strategies for Ischemic Stroke: Recent Insights into Autophagy
- PMID: 35720192
- PMCID: PMC9200548
- DOI: 10.1155/2022/3450207
Novel Therapeutic Strategies for Ischemic Stroke: Recent Insights into Autophagy
Abstract
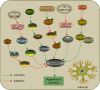
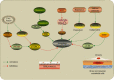
Stroke is one of the leading causes of death and disability worldwide. Autophagy is a conserved cellular catabolic pathway that maintains cellular homeostasis by removal of damaged proteins and organelles, which is critical for the maintenance of energy and function homeostasis of cells. Accumulating evidence demonstrates that autophagy plays important roles in pathophysiological mechanisms under ischemic stroke. Previous investigations show that autophagy serves as a "double-edged sword" in ischemic stroke as it can either promote the survival of neuronal cells or induce cell death in special conditions. Following ischemic stroke, autophagy is activated or inhibited in several cell types in brain, including neurons, astrocytes, and microglia, as well as microvascular endothelial cells, which involves in inflammatory activation, modulation of microglial phenotypes, and blood-brain barrier permeability. However, the exact mechanisms of underlying the role of autophagy in ischemic stroke are not fully understood. This review focuses on the recent advances regarding potential molecular mechanisms of autophagy in different cell types. The focus is also on discussing the "double-edged sword" effect of autophagy in ischemic stroke and its possible underlying mechanisms. In addition, potential therapeutic strategies for ischemic stroke targeting autophagy are also reviewed.
Copyright © 2022 Xiaocheng Lu et al.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

