Homologation of aryl ketones to long-chain ketones and aldehydes via C-C bond cleavage
- PMID: 35720269
- PMCID: PMC9204744
- DOI: 10.1016/j.isci.2022.104505
Homologation of aryl ketones to long-chain ketones and aldehydes via C-C bond cleavage
Abstract
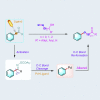
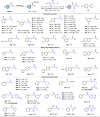
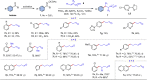
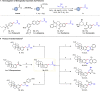
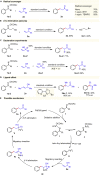
Transition metal-catalyzed C-C bond cleavage is a powerful tool for the reconstruction of a molecular skeleton. We report herein the multi-carbon homologation of aryl ketones to long-chain ketones and aldehydes via ligand-promoted Ar-C(O) bond cleavage and subsequent cross coupling with alkenols. Various (hetero)aryl ketones are compatible in the reaction, affording the corresponding products wtih good to excellent yields with high regioselectivity. Further applications in the late-stage diversification of biologically important molecules demonstrate the synthetic utility of this protocol. Mechanistic studies indicate that the ligand plays an important role in both C-C bond cleavage and the asymmetric migration-insertion process.
Keywords: Chemistry; Organic chemistry; Organic synthesis.
© 2022 The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Aalla S., Gilla G., Metil D.S., Anumula R.R., Vummenthala P.R., Padi P.R. Process improvements of prasugrel hydrochloride: an adenosine diphosphate receptor antagonist. Org. Process Res. Dev. 2012;16:240–243. doi: 10.1021/op200325u. - DOI
-
- Abelman M.M., Overman L.E. Palladium-catalyzed polyene cyclizations of dienyl aryl iodides. J. Am. Chem. Soc. 1988;110:2328–2329. doi: 10.1021/ja00215a068. - DOI
-
- Abelman M.M., Oh T., Overman L.E. Intramolecular alkene arylations for rapid assembly of polycyclic systems containing quaternary centers. A new synthesis of spirooxindoles and other fused and bridged ring systems. J. Org. Chem. 1987;52:4130–4133. doi: 10.1021/jo00227a038. - DOI
-
- Airoldi V., Piccolo O., Roda G., Appiani R., Bavo F., Tassini R., Paganelli S., Arnoldi S., Pallavicini M., Bolchi C. Efficient one-pot reductive aminations of carbonyl compounds with aquivion-Fe as a recyclable catalyst and sodium borohydride. Eur. J. Org. Chem. 2020;2020:162–168. doi: 10.1002/ejoc.201901614. - DOI
LinkOut - more resources
Full Text Sources
Research Materials

