Divergent EGFR/MAPK-Mediated Immune Responses to Clinical Candida Pathogens in Vulvovaginal Candidiasis
- PMID: 35720274
- PMCID: PMC9204526
- DOI: 10.3389/fimmu.2022.894069
Divergent EGFR/MAPK-Mediated Immune Responses to Clinical Candida Pathogens in Vulvovaginal Candidiasis
Abstract
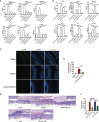
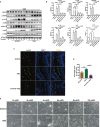
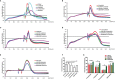
Vulvovaginal candidiasis (VVC) is characterized by symptomatic inflammatory responses in the vagina caused by Candida albicans and non-albicans Candida (NAC) species. The epidermal growth factor receptor (EGFR) -mitogen-activated protein kinase (MAPK) signaling pathway has been linked to immune responses of oral mucosa after C. albicans exposure, but whether this pathway plays a similar response in vaginal epithelial cells is not known. Here, we observed that phosphorylation of EGFR and p38 was continuously activated in vaginal epithelial cells by C. albicans strain SC5314. This differs markedly from oral epithelial cells, which respond in a biphasic manner in order to properly discriminate the morphology of C. albicans. When compared with SC5314, a highly azole-resistant C. albicans isolate 1052 can induce a stronger phosphorylated signal of EGFR and p38, while clinically-isolated NAC strains including C. tropicalis, C. glabrata, C. parapsilosis and C. auris trigger higher levels of phosphorylated ERK1/2 and c-Fos than C. albicans. Inhibition of EGFR significantly reduces inflammatory response and epithelial damage induced by C. albicans both in vitro and in vivo, while inhibition of p38 leads to significant repair of epithelial damage triggered by both C. albicans and NAC species. These results confirm the importance of the EGFR-MAPK signaling in VVC pathogenesis and highlight the remarkable immunogenic differences between C. albicans and NAC species in host-microbe interactions.
Keywords: Candida albicans; EGFR; MAPK; non-albicans Candida species; vaginal epithelial cells; vulvovaginal candidiasis (VVC).
Copyright © 2022 Zhang, Peng, Li, Mei, Yu, Li, She and Liu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

