Regulation of Treg Cell Metabolism and Function in Non-Lymphoid Tissues
- PMID: 35720275
- PMCID: PMC9200993
- DOI: 10.3389/fimmu.2022.909705
Regulation of Treg Cell Metabolism and Function in Non-Lymphoid Tissues
Abstract
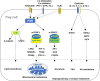
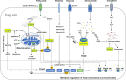
Regulator T cells (Tregs) play pivotal roles in maintaining immune tolerance and regulating immune responses against pathogens and tumors. Reprogramming of cellular metabolism has been determined as a crucial process that connects microenvironmental cues and signaling networks to influence homeostasis and function of tissue Tregs. In adaptation to a variety of non-lymphoid tissues, Tregs coordinate local immune signals and signaling networks to rewire cellular metabolic programs to sustain their suppressive function. Altered Treg metabolism in turn shapes Treg activation and function. In light of the advanced understanding of immunometabolism, manipulation of systemic metabolites has been emerging as an attractive strategy aiming to modulate metabolism and function of tissue Tregs and improve the treatment of immune-related diseases. In this review, we summarize key immune signals and metabolic programs involved in the regulation of tissue Tregs, review the mechanisms underlying the differentiation and function of Tregs in various non-lymphoid tissues, and discuss therapeutic intervention of metabolic modulators of tissue Tregs for the treatment of autoimmune diseases and cancer.
Keywords: Treg function; Treg homeostasis; Treg metabolism; metabolic signaling; tissue Treg cells.
Copyright © 2022 Yang.
Conflict of interest statement
The authors declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Regulatory T cell metabolism at the intersection between autoimmune diseases and cancer.Eur J Immunol. 2020 Nov;50(11):1626-1642. doi: 10.1002/eji.201948470. Epub 2020 Oct 26. Eur J Immunol. 2020. PMID: 33067808 Free PMC article. Review.
-
Immunometabolic Checkpoints of Treg Dynamics: Adaptation to Microenvironmental Opportunities and Challenges.Front Immunol. 2019 Aug 27;10:1889. doi: 10.3389/fimmu.2019.01889. eCollection 2019. Front Immunol. 2019. PMID: 31507585 Free PMC article. Review.
-
Emerging Functions of Regulatory T Cells in Tissue Homeostasis.Front Immunol. 2018 Apr 25;9:883. doi: 10.3389/fimmu.2018.00883. eCollection 2018. Front Immunol. 2018. PMID: 29887862 Free PMC article. Review.
-
Navigating the metabolic landscape of regulatory T cells: from autoimmune diseases to tumor microenvironments.Curr Opin Immunol. 2025 Feb;92:102511. doi: 10.1016/j.coi.2024.102511. Epub 2024 Dec 13. Curr Opin Immunol. 2025. PMID: 39674060 Review.
-
Regulatory T cells in peripheral tissue tolerance and diseases.Front Immunol. 2023 May 1;14:1154575. doi: 10.3389/fimmu.2023.1154575. eCollection 2023. Front Immunol. 2023. PMID: 37197653 Free PMC article. Review.
Cited by
-
Tumor growth inhibition and immune system activation following treatment with thorium-227 conjugates and PD-1 check-point inhibition in the MC-38 murine model.Front Med (Lausanne). 2022 Nov 15;9:1033303. doi: 10.3389/fmed.2022.1033303. eCollection 2022. Front Med (Lausanne). 2022. PMID: 36457578 Free PMC article.
-
BATF sustains homeostasis and functionality of bone marrow Treg cells to preserve homeostatic regulation of hematopoiesis and development of B cells.Front Immunol. 2023 Feb 22;14:1026368. doi: 10.3389/fimmu.2023.1026368. eCollection 2023. Front Immunol. 2023. PMID: 36911703 Free PMC article.
-
Proteobacteria impair anti-tumor immunity in the omentum by consuming arginine.Cell Host Microbe. 2024 Jul 10;32(7):1177-1191.e7. doi: 10.1016/j.chom.2024.06.003. Epub 2024 Jun 27. Cell Host Microbe. 2024. PMID: 38942027 Free PMC article.
-
Interactions between gut microbiota and Parkinson's disease: The role of microbiota-derived amino acid metabolism.Front Aging Neurosci. 2022 Nov 2;14:976316. doi: 10.3389/fnagi.2022.976316. eCollection 2022. Front Aging Neurosci. 2022. PMID: 36408101 Free PMC article. Review.
-
Causal relationships between immune cells, inflammatory cytokines, and pertussis: Bidirectional 2-sample Mendelian randomization study and mediation analysis.Medicine (Baltimore). 2024 Nov 29;103(48):e40712. doi: 10.1097/MD.0000000000040712. Medicine (Baltimore). 2024. PMID: 39612418 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

