Iguratimod Restrains Circulating Follicular Helper T Cell Function by Inhibiting Glucose Metabolism via Hif1α-HK2 Axis in Rheumatoid Arthritis
- PMID: 35720293
- PMCID: PMC9199372
- DOI: 10.3389/fimmu.2022.757616
Iguratimod Restrains Circulating Follicular Helper T Cell Function by Inhibiting Glucose Metabolism via Hif1α-HK2 Axis in Rheumatoid Arthritis
Abstract
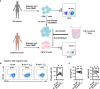
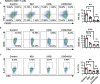
Iguratimod (IGU) is a novel disease modified anti-rheumatic drug, which has been found to act directly on B cells for inhibiting the production of antibodies in rheumatoid arthritis (RA) patients. Follicular helper T (Tfh) cells, a key T cell subsets in supporting B cell differentiation and antibody production, have been shown to play critical roles in RA. However, whether IGU can inhibit RA Tfh cells which further restrains B cell function remains unclear. Here, we aimed to explore the roles of IGU in regulating RA circulating Tfh (cTfh) cell function and investigate the potential mechanism associated with cell glucose metabolism. In our study, we found that IGU could act on RA-CD4+ T cells to reduce T cell-dependent antibody production. IGU decreased the percentage of RA cTfh cells and the expression of Tfh cell-related molecules and cytokines which were involved in B cell functions. Importantly, our data showed that IGU significantly restrained the cTfh cell function by inhibiting glucose metabolism, which relied on Hif1α-HK2 axis. In summary, we clarified a new target and mechanism of IGU by restraining RA cTfh cell function via inhibiting Hif1α-HK2-glucose metabolism axis. Our study demonstrates the potential application of IGU in the treatment of diseases related to abnormal metabolism and function of Tfh cells.
Keywords: Hif1α-HK2 axis; circulating follicular helper T cells; glucose metabolism; iguratimod; rheumatoid arthritis.
Copyright © 2022 Bai, Lu, Liu, Tang, Ye, Jin, Wang and Li.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

