Post-Transplant Thrombotic Microangiopathy due to a Pathogenic Mutation in Complement Factor I in a Patient With Membranous Nephropathy: Case Report and Review of Literature
- PMID: 35720299
- PMCID: PMC9204634
- DOI: 10.3389/fimmu.2022.909503
Post-Transplant Thrombotic Microangiopathy due to a Pathogenic Mutation in Complement Factor I in a Patient With Membranous Nephropathy: Case Report and Review of Literature
Abstract
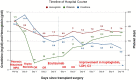
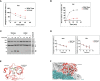
Thrombotic microangiopathy (TMA) is characterized by microangiopathic hemolytic anemia, thrombocytopenia and organ injury occurring due to endothelial cell damage and microthrombi formation in small vessels. TMA is primary when a genetic or acquired defect is identified, as in atypical hemolytic uremic syndrome (aHUS) or secondary when occurring in the context of another disease process such as infection, autoimmune disease, malignancy or drugs. Differentiating between a primary complement-mediated process and one triggered by secondary factors is critical to initiate timely treatment but can be challenging for clinicians, especially after a kidney transplant due to presence of multiple confounding factors. Similarly, primary membranous nephropathy is an immune-mediated glomerular disease associated with circulating autoantibodies (directed against the M-type phospholipase A2 receptor (PLA2R) in 70% cases) while secondary membranous nephropathy is associated with infections, drugs, cancer, or other autoimmune diseases. Complement activation has also been proposed as a possible mechanism in the etiopathogenesis of primary membranous nephropathy; however, despite complement being a potentially common link, aHUS and primary membranous nephropathy have not been reported together. Herein we describe a case of aHUS due to a pathogenic mutation in complement factor I that developed after a kidney transplant in a patient with an underlying diagnosis of PLA2R antibody associated-membranous nephropathy. We highlight how a systematic and comprehensive analysis helped to define the etiology of aHUS, establish mechanism of disease, and facilitated timely treatment with eculizumab that led to recovery of his kidney function. Nonetheless, ongoing anti-complement therapy did not prevent recurrence of membranous nephropathy in the allograft. To our knowledge, this is the first report of a patient with primary membranous nephropathy and aHUS after a kidney transplant.
Keywords: atypical hemolytic uremic syndrome; complement factor I; complement functional analysis; kidney transplantation; membranous nephropathy; thrombotic microangiopathy.
Copyright © 2022 Saleem, Shaikh, Hu, Pozzi and Java.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

