Ferroptosis and Autoimmune Diseases
- PMID: 35720308
- PMCID: PMC9203688
- DOI: 10.3389/fimmu.2022.916664
Ferroptosis and Autoimmune Diseases
Abstract
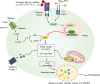
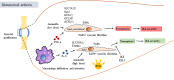
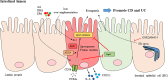
Adequate control of autoimmune diseases with an unclear etiology resulting from autoreactivation of the immune system remains a major challenge. One of the factors that trigger autoimmunity is the abnormal induction of cell death and the inadequate clearance of dead cells that leads to the exposure or release of intracellular contents that activate the immune system. Different from other cell death subtypes, such as apoptosis, necroptosis, autophagy, and pyroptosis, ferroptosis has a unique association with the cellular iron load (but not the loads of other metals) and preserves its distinguishable morphological, biological, and genetic features. This review addresses how ferroptosis is initiated and how it contributes to the pathogenesis of autoimmune diseases, including systemic lupus erythematosus, rheumatoid arthritis, and inflammatory bowel diseases. The mechanisms responsible for ferroptosis-associated events are discussed. We also cover the perspective of targeting ferroptosis as a potential therapeutic for patients with autoimmune diseases. Collectively, this review provides up-to-date knowledge regarding how ferroptosis occurs and its significance in autoimmune diseases.
Keywords: ferroptosis; inflammation; inflammatory bowel diseases; rheumatoid arthritis; systemic lupus erythematosus.
Copyright © 2022 Lai, Wu, Wu, Luo and Lai.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

