Low Levels of Factor H Family Proteins During Meningococcal Disease Indicate Systemic Processes Rather Than Specific Depletion by Neisseria meningitidis
- PMID: 35720329
- PMCID: PMC9204383
- DOI: 10.3389/fimmu.2022.876776
Low Levels of Factor H Family Proteins During Meningococcal Disease Indicate Systemic Processes Rather Than Specific Depletion by Neisseria meningitidis
Abstract
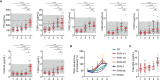
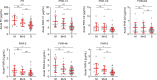
Neisseria meningitidis, the causative agent of meningococcal disease (MD), evades complement-mediated clearance upon infection by 'hijacking' the human complement regulator factor H (FH). The FH protein family also comprises the homologous FH-related (FHR) proteins, hypothesized to act as antagonists of FH, and FHR-3 has recently been implicated to play a major role in MD susceptibility. Here, we show that the circulating levels of all FH family proteins, not only FH and FHR-3, are equally decreased during the acute illness. We did neither observe specific consumption of FH or FHR-3 by N. meningitidis, nor of any of the other FH family proteins, suggesting that the globally reduced levels are due to systemic processes including dilution by fluid administration upon admission and vascular leakage. MD severity associated predominantly with a loss of FH rather than FHRs. Additionally, low FH levels associated with renal failure, suggesting insufficient protection of host tissue by the active protection by the FH protein family, which is reminiscent of reduced FH activity in hemolytic uremic syndrome. Retaining higher levels of FH may thus limit tissue injury during MD.
Keywords: FHR; Neisseria meningitidis; complement; factor H; meningococcal disease.
Copyright © 2022 van Beek, Pouw, Wright, Sallah, Inwald, Hoggart, Brouwer, Galassini, Thomas, Calvo-Bado, Fink, Jongerius, Hibberd, Wouters, Levin and Kuijpers.
Conflict of interest statement
RP, MB, DW and TK are co-inventors of patents describing the potentiation of FH with monoclonal antibodies and therapeutic uses thereof. JT, LCB and CF are employed by Micropathology Ltd. All other authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Interaction of Shiga toxin 2 with complement regulators of the factor H protein family.Mol Immunol. 2014 Mar;58(1):77-84. doi: 10.1016/j.molimm.2013.11.009. Epub 2013 Dec 5. Mol Immunol. 2014. PMID: 24317278
-
Binding of complement factor H (fH) to Neisseria meningitidis is specific for human fH and inhibits complement activation by rat and rabbit sera.Infect Immun. 2009 Feb;77(2):764-9. doi: 10.1128/IAI.01191-08. Epub 2008 Dec 1. Infect Immun. 2009. PMID: 19047406 Free PMC article.
-
Variation in CFHR3 determines susceptibility to meningococcal disease by controlling factor H concentrations.Am J Hum Genet. 2022 Sep 1;109(9):1680-1691. doi: 10.1016/j.ajhg.2022.08.001. Epub 2022 Aug 24. Am J Hum Genet. 2022. PMID: 36007525 Free PMC article.
-
Contribution of functional and quantitative genetic variants of Complement Factor H and Factor H-Related (FHR) proteins on renal pathology.Nefrologia (Engl Ed). 2022 May-Jun;42(3):280-289. doi: 10.1016/j.nefroe.2022.09.002. Epub 2022 Sep 22. Nefrologia (Engl Ed). 2022. PMID: 36154806 Review.
-
Human genetics of meningococcal infections.Hum Genet. 2020 Jun;139(6-7):961-980. doi: 10.1007/s00439-020-02128-4. Epub 2020 Feb 17. Hum Genet. 2020. PMID: 32067109 Free PMC article. Review.
Cited by
-
Mechanisms by which Factor H protects Trypanosoma cruzi from the alternative pathway of complement.Front Immunol. 2024 Feb 1;15:1152000. doi: 10.3389/fimmu.2024.1152000. eCollection 2024. Front Immunol. 2024. PMID: 38361922 Free PMC article.
-
Factor H-related 2 levels dictate FHR dimer composition.Sci Rep. 2025 Mar 28;15(1):10669. doi: 10.1038/s41598-025-94064-4. Sci Rep. 2025. PMID: 40148491 Free PMC article.
-
The human factor H protein family - an update.Front Immunol. 2024 Feb 12;15:1135490. doi: 10.3389/fimmu.2024.1135490. eCollection 2024. Front Immunol. 2024. PMID: 38410512 Free PMC article. Review.
References
-
- Borrow R, Alarcón P, Carlos J, Caugant DA, Christensen H, Debbag R, et al. . The Global Meningococcal Initiative: Global Epidemiology, the Impact of Vaccines on Meningococcal Disease and the Importance of Herd Protection. Expert Rev Vaccines (2016) 16:313–28. doi: 10.1080/14760584.2017.1258308 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

