Changes in Systemic Regulatory T Cells, Effector T Cells, and Monocyte Populations Associated With Early-Life Stunting
- PMID: 35720335
- PMCID: PMC9202423
- DOI: 10.3389/fimmu.2022.864084
Changes in Systemic Regulatory T Cells, Effector T Cells, and Monocyte Populations Associated With Early-Life Stunting
Abstract
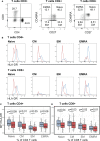
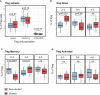
Stunting and environmental enteric dysfunction (EED) may be responsible for altered gut and systemic immune responses. However, their impact on circulating immune cell populations remains poorly characterized during early life. A detailed flow cytometry analysis of major systemic immune cell populations in 53 stunted and 52 non-stunted (2 to 5 years old) children living in Antananarivo (Madagascar) was performed. Compared to age-matched non-stunted controls, stunted children aged 2-3 years old had a significantly lower relative proportion of classical monocytes. No significant associations were found between stunting and the percentages of effector T helper cell populations (Th1, Th2, Th17, Th1Th17, and cTfh). However, we found that HLA-DR expression (MFI) on all memory CD4+ or CD8+ T cell subsets was significantly lower in stunted children compared to non-stunted controls. Interestingly, in stunted children compared to the same age-matched non-stunted controls, we observed statistically significant age-specific differences in regulatory T cells (Treg) subsets. Indeed, in 2- to 3-year-old stunted children, a significantly higher percentage of memory Treg, whilst a significantly lower percentage of naive Treg, was found. Our results revealed that both innate and adaptive systemic cell percentages, as well as activation status, were impacted in an age-related manner during stunting. Our study provides valuable insights into the understanding of systemic immune system changes in stunted children.
Keywords: Madagascar; environmental enteric dysfunction; flow cytometry; monocytes; regulatory T cells; stunting; systemic immune cells.
Copyright © 2022 Andriamanantena, Randrianarisaona, Rakotondrainipiana, Andriantsalama, Randriamparany, Randremanana, Randrianirina, Novault, Duffy, Huetz, Hasan, Schoenhals, Sansonetti, Vonaesch, Vigan-Womas and Afribiota Investigators.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- UNICEF, WHO, World Bank . Levels and Trends in Child Malnutrition: Key Findings of the 2020 Edition of the Joint Child Malnutrition Estimates Vol. 24. . Geneva: World Health Organization; (2020) p. 1–16. Available at: https://www.who.int/publications/i/item/jme-2020-edition.
-
- Hoest C, Seidman JC, Pan W, Ambikapathi R, Kang G, Kosek M, et al. . Evaluating Associations Between Vaccine Response and Malnutrition, Gut Function, and Enteric Infections in the MAL-ED Cohort Study: Methods and Challenges. Clin Infect Dis (2014) 59(Suppl 4):S273–9. doi: 10.1093/cid/ciu611 - DOI - PMC - PubMed
-
- Kirkpatrick BD, Colgate ER, Mychaleckyj JC, Haque R, Dickson DM, Carmolli MP, et al. . The “Performance of Rotavirus and Oral Polio Vaccines in Developing Countries” (PROVIDE) Study: Description of Methods of an Interventional Study Designed to Explore Complex Biologic Problems. Am J Trop Med Hyg (2015) 92(4):744–51. doi: 10.4269/ajtmh.14-0518 - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials

