Adiponectin Ameliorates GMH-Induced Brain Injury by Regulating Microglia M1/M2 Polarization Via AdipoR1/APPL1/AMPK/PPARγ Signaling Pathway in Neonatal Rats
- PMID: 35720361
- PMCID: PMC9203698
- DOI: 10.3389/fimmu.2022.873382
Adiponectin Ameliorates GMH-Induced Brain Injury by Regulating Microglia M1/M2 Polarization Via AdipoR1/APPL1/AMPK/PPARγ Signaling Pathway in Neonatal Rats
Abstract
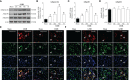
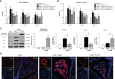
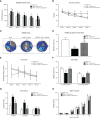
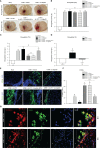
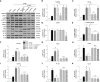
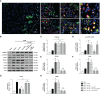
Adiponectin (APN), a fat-derived plasma hormone, is a classic anti-inflammatory agent. Multiple studies have demonstrated the beneficial role of APN in acute brain injury, but the effect of APN in germinal matrix hemorrhage (GMH) is unclear, and the underlying molecular mechanisms remain largely undefined. In the current study, we used a GMH rat model with rh-APN treatment, and we observed that APN demonstrated a protective effect on neurological function and an inhibitory effect on neuroinflammation after GMH. To further explore the underlying mechanisms of these effects, we found that the expression of Adiponectin receptor 1 (AdipoR1) primarily colocalized with microglia and neurons in the brain. Moreover, AdiopR1, but not AdipoR2, was largely increased in GMH rats. Meanwhile, further investigation showed that APN treatment promoted AdipoR1/APPL1-mediated AMPK phosphorylation, further increased peroxisome proliferator-activated receptor gamma (PPARγ) expression, and induced microglial M2 polarization to reduce the neuroinflammation and enhance hematoma resolution in GMH rats. Importantly, either knockdown of AdipoR1, APPL1, or LKB1, or specific inhibition of AMPK/PPARγ signaling in microglia abrogated the protective effect of APN after GMH in rats. In all, we propose that APN works as a potential therapeutic agent to ameliorate the inflammatory response following GMH by enhancing the M2 polarization of microglia via AdipoR1/APPL1/AMPK/PPARγ signaling pathway, ultimately attenuating inflammatory brain injury induced by hemorrhage.
Keywords: GMH; adiponectin; hematoma resolution; microglial polarization; neuroinflammation.
Copyright © 2022 Xu, Li, Weng, Wei, He, Doycheva, Lenahan, Tang, Zhou, Liu, Xu, Liu, He, Tang, Zhang and Duan.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

