Antibody Response to COVID-19 Booster Vaccination in Healthcare Workers
- PMID: 35720366
- PMCID: PMC9205631
- DOI: 10.3389/fimmu.2022.872667
Antibody Response to COVID-19 Booster Vaccination in Healthcare Workers
Abstract
Objective: To evaluate the mean increase of anti-S IgG antibody titer between the basal, pre-booster level to the titer assessed 14 days after the booster dose of BNT162b2.
Patients and methods: The RENAISSANCE study is an observational, longitudinal, prospective, population-based study, conducted on healthcare workers of Niguarda Hospital in Milan, Italy who received a BNT162b2 booster dose at least 180 days after their second dose or after positivity for SARS-CoV-2 and accepted to take part in the study. The RENAISSANCE study was conducted from January 1, 2021 through December 28, 2021.
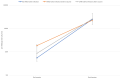
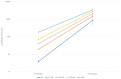
Findings: 1,738 subjects were enrolled among healthcare workers registered for the booster administration at our hospital. Overall, 0.4% of subjects were seronegative at the pre-booster evaluation, and 1 subject had a titer equal to 50 AU/ml: none of the evaluated subjects was seronegative after the booster dose. Thus, the efficacy of the booster in our population was universal. Mean increase of pre- to post-booster titer was more significant in subjects who never had SARS-CoV-2 (44 times CI 95% 42-46) compared to those who had it, before (33 times, CI 95% 13-70) or after the first vaccination cycle (12 times, CI 95% 11-14). Differently from sex, age and pre-booster titers affected the post-booster antibody response. Nevertheless, the post-booster titer was very similar in all subgroups, and independent of a prior exposure to SARS-CoV-2, pre-booster titer, sex or age.
Conclusion: Our study shows a potent universal antibody response of the booster dose of BNT162b2, regardless of pre-booster vaccine seronegativity.
Keywords: COVID-19; SARS-CoV-2; antibody response; healthcare workers (HCWs); vaccine booster.
Copyright © 2022 Pani, Romandini, Schianchi, Senatore, Gagliardi, Gazzaniga, Agliardi, Conti, Schenardi, Maggi, D’Onghia, Panetta, Renica, Molteni, Vismara, Campisi, Bertuzzi, Giroldi, Zoppini, Moreno, Merli, Bosio, Puoti and Scaglione.
Conflict of interest statement
Author Valentina Panetta is employed by L’altrastatistica srl – Consultancy & Training, Rome, Italy. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Covariants. Available at: https://covariants.org/.
-
- Cov Lineages - Omicron Variant. Available at: https://cov-lineages.org/global_report_BA.1.html.
-
- Puranik A, Lenehan PJ, Silvert E, Niesen MJM, Corchado-Garcia J, O'Horo JC,, et al. . Comparison of Two Highly-Effective MRNA Vaccines for COVID-19 During Periods of Alpha and Delta Variant Prevalence. medRxiv [Preprint] (2021). doi: 10.1101/2021.08.06.21261707 - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

