Neutrophils in COVID-19: Not Innocent Bystanders
- PMID: 35720378
- PMCID: PMC9199383
- DOI: 10.3389/fimmu.2022.864387
Neutrophils in COVID-19: Not Innocent Bystanders
Abstract
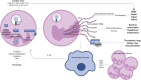
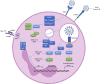
Unusually for a viral infection, the immunological phenotype of severe COVID-19 is characterised by a depleted lymphocyte and elevated neutrophil count, with the neutrophil-to-lymphocyte ratio correlating with disease severity. Neutrophils are the most abundant immune cell in the bloodstream and comprise different subpopulations with pleiotropic actions that are vital for host immunity. Unique neutrophil subpopulations vary in their capacity to mount antimicrobial responses, including NETosis (the generation of neutrophil extracellular traps), degranulation and de novo production of cytokines and chemokines. These processes play a role in antiviral immunity, but may also contribute to the local and systemic tissue damage seen in acute SARS-CoV-2 infection. Neutrophils also contribute to complications of COVID-19 such as thrombosis, acute respiratory distress syndrome and multisystem inflammatory disease in children. In this Progress review, we discuss the anti-viral and pathological roles of neutrophils in SARS-CoV-2 infection, and potential therapeutic strategies for COVID-19 that target neutrophil-mediated inflammatory responses.
Keywords: COVID-19; SARS-CoV-2; inflammation; innate immunity; neutrophil.
Copyright © 2022 McKenna, Wubben, Isaza-Correa, Melo, Mhaonaigh, Conlon, O’Donnell, Ní Cheallaigh, Hurley, Stevenson, Little and Molloy.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
Publication types
MeSH terms
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

