High Salt Induces a Delayed Activation of Human Neutrophils
- PMID: 35720394
- PMCID: PMC9204211
- DOI: 10.3389/fimmu.2022.831844
High Salt Induces a Delayed Activation of Human Neutrophils
Abstract
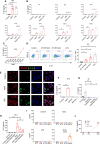
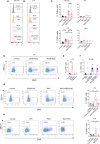
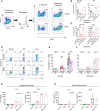
High salt (NaCl) concentrations are found in a number of tissues under physiological and pathological conditions. Here, we analyzed the effects induced by high salt on the function of human neutrophils. The culture of neutrophils in medium supplemented with high salt (50 mM NaCl) for short periods (30-120 min) inhibited the ability of conventional agonists to induce the production of IL-8 and the activation of respiratory burst. By contrast, exposure to high salt for longer periods (6-18 h) resulted in the activation of neutrophils revealed by the production of high levels of IL-8, the activation of the respiratory burst, and a marked synergistic effect on the production of TNF-α induced by LPS. Increasing osmolarity of the culture medium by the addition of sorbitol or mannitol (100 mM) was shown to be completely unable to stimulate neutrophil responses, suggesting that high sodium but not an increased osmolarity mediates the activation on neutrophils responses. A similar biphasic effect was observed when the function of monocytes was analyzed. Short term exposure to high salt suppressed IL-8 and TNF-α production induced by LPS while culture for longer periods triggered the production of IL-8 but not TNF-α in the absence of LPS stimulation. Contradictory results have been published regarding how high salt modulates neutrophil function. Our results suggest that the modulation of neutrophil function by high salt is strongly dependent on the exposure time.
Keywords: IL-8 (CXCL8); inflammation; innate immunity; oxygen reactive intermediates; sodium chloride.
Copyright © 2022 Mazzitelli, Bleichmar, Melucci, Gerber, Toscanini, Cuestas, Diaz and Geffner.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

