Nogo-A/NgR signaling regulates stemness in cancer stem-like cells derived from U87MG glioblastoma cells
- PMID: 35720478
- PMCID: PMC9185138
- DOI: 10.3892/ol.2022.13351
Nogo-A/NgR signaling regulates stemness in cancer stem-like cells derived from U87MG glioblastoma cells
Abstract
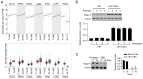
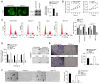
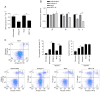
Neurite outgrowth inhibitor A (Nogo-A), a member of the reticulon 4 family, is an axon regeneration inhibitor that is negatively associated with the malignancy of oligodendroglial tumors. It has been suggested that the Nogo-A/Nogo Receptor (NgR) pathway plays a promoting effect in regulating cancer stem-like cells (CSCs) derived from glioblastoma, indicating that Nogo-A could exert different roles in CSCs than those in parental cancer cells. In the present study, CSCs were generated from the human Uppsala 87 malignant glioma (U87MG) cell line. These U87MG-CSCs were characterized by the upregulation of CD44 and CD133, which are two markers of stemness. The expression levels of Nogo-A and the differentiation of U87MG-CSCs were investigated. In addition, the proliferation, invasion and colony formation U87MG-CSCs were examined. Using culture in serum-containing medium, U87MG-CSCs were differentiated into neuron-like cells specifically expressing MAP2, β-III-tubulin and nestin. Nogo-A was upregulated in U87MG-CSCs compared with parental cells. Knockdown of Nogo-A and inhibition of the Nogo-A/NgR signaling pathway in U87MG-CSCs markedly decreased cell viability, cell cycle entry, invasion and tumor formation, indicating that Nogo-A could regulate U87MG-CSC function. Moreover, Nogo-A was involved in intracellular ATP synthesis and scavenging of accumulated reactive oxygen species. Nogo-A/NgR pathway exerted protective effects against hypoxia-induced non-apoptotic and apoptotic cell death. These results suggest that Nogo-A plays an important role in regulating U87MG-CSCs via the Nogo-A/NgR signaling pathway. Nogo-A may also different roles in U87MG-CSCs compared with their parental cells.
Keywords: Nogo-A; apoptosis; cancer stem-like cells; glioblastoma; malignancy.
Copyright: © Ai et al.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
