Evaluation of P16/Ki67 (CINtecPlus) and L1-capsid compared with HPV-genotyping in cervical cytology in women ≥35 years old focusing on patients with atypical squamous cells of undetermined significance
- PMID: 35720497
- PMCID: PMC9185144
- DOI: 10.3892/ol.2022.13362
Evaluation of P16/Ki67 (CINtecPlus) and L1-capsid compared with HPV-genotyping in cervical cytology in women ≥35 years old focusing on patients with atypical squamous cells of undetermined significance
Abstract
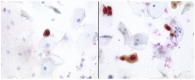
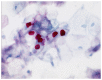
Cervical cancer is the third most common cancer in women worldwide. Conventional cytological examination as a screening method with Papanicolaou has been established to reduce the incidence of dysplasia and cervical cancer for years. In addition to the conventional screening, the introduction of immunocytochemical examinations, including CINtecPlus and L1-capsid, has been demonstrated to have a positive impact on screening results. In addition to morphological screening methods, human papillomavirus (HPV)-testing has also been demonstrated to possess an enormous potential in the cervical screening process. Additionally, different screening models ranging from conventional cytological screening to primary HPV-testing do exist in different countries. At the beginning of the year 2020, a combination of cytological screening and HPV-testing was introduced in Germany for women ≥35 years. The aim of the present study was to evaluate the role of morphological screening, including immunocytochemistry, and to compare it with HPV-genotyping. Immunocytochemistry was added to confirm the diagnosis but needs established infrastructure and well-trained personnel. Furthermore, there was a need to establish the HPV-screening method. In the Institute for Pathology and Cytology (Schuettorf, Leer, Germany), 146,800 samples of women (>35 years old) were examined between January 2020 and January 2021. The present study retrospectively analyzed 146,800 samples. Each sample was examined using a conventional cytological technique and HPV-high risk-Test (HPV-HR-Test) with Viper-BD. Immunocytochemistry with CINtecPlus and L1-capsid was added in some cases. A total of 555 cases were cytological diagnosed as atypical squamous cells of undetermined significance (ASC-US; IIp). After performing immunocytochemistry, 79% of cases were suspected to be positive and 1.48% of cases were definitely positive. The HPV-HR-Test was positive in 26.4% of cases. Among cases of ASC-US and HPV-HR-negativity, 33.7% were suspicious of immunocytochemical positivity and 0.5% were definitely positive. Among patients with HPV-16-negativity, 13.6% were patients with highly squamous intraepithelial lesion (HSIL) and 22.7% were patients with low-grade squamous intraepithelial lesion (LSIL) and HSIL. Among patients with HPV-18-negativity, 14.3% were patients with HSIL and 19.5% were patients with LSIL and HSIL. There were 107 cases in this group of cases with negativity of both HPV-16 and HPV-18. After performing the colposcopy and biopsy, there were 6.5% with cervical intra-epithelial neoplasia (CIN) I, 8.4% with CIN II and 5.6% with CIN III. In conclusion, there is still a need for conventional cytological examination and maybe the addition of immunocytochemistry to confirm the diagnosis and to exclude dysplasia of cervical epithelium. The HPV-HR-Test is not enough as a screening method and may be misleading.
Keywords: CINtecPlus; L1-capsid; atypical squamous cells of undetermined significance; cervical cytology.
Copyright: © Abbas et al.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


Similar articles
-
HPV-genotyping versus conventional cervical cytology as a screening method to detect dysplastic cervical epithelial changes.Sci Rep. 2022 Oct 24;12(1):17828. doi: 10.1038/s41598-022-22438-z. Sci Rep. 2022. PMID: 36280748 Free PMC article.
-
Role of Cervical Cancer Biomarkers p16 and Ki67 in Abnormal Cervical Cytological Smear.J Obstet Gynaecol India. 2021 Feb;71(1):72-77. doi: 10.1007/s13224-020-01380-y. Epub 2020 Nov 18. J Obstet Gynaecol India. 2021. PMID: 33814802 Free PMC article.
-
[Verification of doubtful PAP smear results of women included in the screening program in the Podlaskie province].Ginekol Pol. 2013 Aug;84(8):691-5. doi: 10.17772/gp/1625. Ginekol Pol. 2013. PMID: 24191502 Polish.
-
Overview of human papillomavirus-based and other novel options for cervical cancer screening in developed and developing countries.Vaccine. 2008 Aug 19;26 Suppl 10:K29-41. doi: 10.1016/j.vaccine.2008.06.019. Vaccine. 2008. PMID: 18847555 Review.
-
Screening for cervical cancer: new alternatives and research.Salud Publica Mex. 2003;45 Suppl 3:S376-87. doi: 10.1590/s0036-36342003000900012. Salud Publica Mex. 2003. PMID: 14746031 Review.
Cited by
-
A Prospective Study on the Progression, Recurrence, and Regression of Cervical Lesions: Assessing Various Screening Approaches.J Clin Med. 2024 Feb 28;13(5):1368. doi: 10.3390/jcm13051368. J Clin Med. 2024. PMID: 38592206 Free PMC article.
-
Role of L1 HPV Protein Expression in the Cytological Diagnosis of Precancerous Cervical Lesions.J Clin Med. 2025 May 12;14(10):3376. doi: 10.3390/jcm14103376. J Clin Med. 2025. PMID: 40429372 Free PMC article. Review.
-
HPV-genotyping versus conventional cervical cytology as a screening method to detect dysplastic cervical epithelial changes.Sci Rep. 2022 Oct 24;12(1):17828. doi: 10.1038/s41598-022-22438-z. Sci Rep. 2022. PMID: 36280748 Free PMC article.
References
-
- Robert Koch Institute, corp-author. Epidemiology. Vol. 8. Berlin: 2012. Malignancy in Germany 2007/2008.
LinkOut - more resources
Full Text Sources
Miscellaneous
