Target Values and Daytime Variation of Bone Turnover Markers in Monitoring Osteoporosis Treatment After Fractures
- PMID: 35720666
- PMCID: PMC9189911
- DOI: 10.1002/jbm4.10633
Target Values and Daytime Variation of Bone Turnover Markers in Monitoring Osteoporosis Treatment After Fractures
Abstract
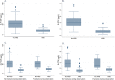
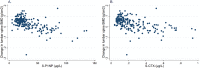
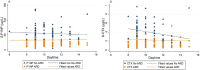
The serum bone turnover markers (BTM) procollagen type 1 N-terminal propeptide (P1NP) and C-terminal cross-linking telopeptide of type 1 collagen (CTX) are recommended for monitoring adherence and response of antiresorptive drugs (ARD). BTM are elevated about 1 year after fracture and therefore BTM target values are most convenient in ARD treatment follow-up of fracture patients. In this prospective cohort study, we explored the cut-off values of P1NP and CTX showing the best discriminating ability with respect to adherence and treatment effects, reflected in bone mineral density (BMD) changes. Furthermore, we explored the ability of BTM to predict subsequent fractures and BTM variation during daytime in patients using ARD or not. After a fragility fracture, 228 consenting patients (82.2% women) were evaluated for ARD indication and followed for a mean of 4.6 years (SD 0.5 years). BMD was measured at baseline and after 2 years. Serum BTM were measured after 1 or 2 years. The largest area under the curve (AUC) for discrimination of patients taking ARD or not was shown for P1NP <30 μg/L and CTX <0.25 μg/L. AUC for discrimination of patients with >2% gain in BMD (lumbar spine and total hip) was largest at cut-off values for P1NP <30 μg/L and CTX <0.25 μg/L. Higher P1NP was associated with increased fracture risk in patients using ARD (hazard ratio [HR]logP1NP = 15.0; 95% confidence interval [CI] 2.7-83.3), p = 0.002. P1NP and CTX were stable during daytime, except in those patients not taking ARD, where CTX decreased by 21% per hour during daytime. In conclusion, P1NP <30 μg/L and CTX <0.25 μg/L yield the best discrimination between patients taking and not taking ARD and the best prediction of BMD gains after 2 years. Furthermore, higher P1NP is associated with increased fracture risk in patients on ARD. BTM can be measured at any time during the day in patients on ARD. © 2022 The Authors. JBMR Plus published by Wiley Periodicals LLC on behalf of American Society for Bone and Mineral Research.
Keywords: ANTIRESORPTIVES; BIOCHEMICAL MARKERS OF BONE TURNOVER; DXA; FRACTURE RISK ASSESSMENT; OSTEOPOROSIS.
© 2022 The Authors. JBMR Plus published by Wiley Periodicals LLC on behalf of American Society for Bone and Mineral Research.
Figures

References
-
- Eastell R, Szulc P. Use of bone turnover markers in postmenopausal osteoporosis. Lancet Diabetes Endocrinol. 2017;5(11):908‐923. - PubMed
-
- Fontalis A, Eastell R. The challenge of long‐term adherence: the role of bone turnover markers in monitoring bisphosphonate treatment of osteoporosis. Bone. 2020;136:115336. - PubMed
-
- Vasikaran S, Cooper C, Eastell R, et al. International Osteoporosis Foundation and International Federation of Clinical Chemistry and Laboratory Medicine position on bone marker standards in osteoporosis. Clin Chem Lab Med. 2011;49(8):1271‐1274. - PubMed
-
- Vasikaran S, Eastell R, Bruyere O, et al. Markers of bone turnover for the prediction of fracture risk and monitoring of osteoporosis treatment: a need for international reference standards. Osteoporos Int. 2011;22(2):391‐420. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
