Automatic Detection of High-Frequency Oscillations With Neuromorphic Spiking Neural Networks
- PMID: 35720714
- PMCID: PMC9205405
- DOI: 10.3389/fnins.2022.861480
Automatic Detection of High-Frequency Oscillations With Neuromorphic Spiking Neural Networks
Abstract
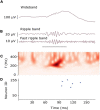
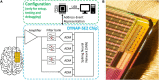
Interictal high-frequency oscillations (HFO) detected in electroencephalography recordings have been proposed as biomarkers of epileptogenesis, seizure propensity, disease severity, and treatment response. Automatic HFO detectors typically analyze the data offline using complex time-consuming algorithms, which limits their clinical application. Neuromorphic circuits offer the possibility of building compact and low-power processing systems that can analyze data on-line and in real time. In this review, we describe a fully automated detection pipeline for HFO that uses, for the first time, spiking neural networks and neuromorphic technology. We demonstrated that our HFO detection pipeline can be applied to recordings from different modalities (intracranial electroencephalography, electrocorticography, and scalp electroencephalography) and validated its operation in a custom-designed neuromorphic processor. Our HFO detection approach resulted in high accuracy and specificity in the prediction of seizure outcome in patients implanted with intracranial electroencephalography and electrocorticography, and in the prediction of epilepsy severity in patients recorded with scalp electroencephalography. Our research provides a further step toward the real-time detection of HFO using compact and low-power neuromorphic devices. The real-time detection of HFO in the operation room may improve the seizure outcome of epilepsy surgery, while the use of our neuromorphic processor for non-invasive therapy monitoring might allow for more effective medication strategies to achieve seizure control. Therefore, this work has the potential to improve the quality of life in patients with epilepsy by improving epilepsy diagnostics and treatment.
Keywords: electroencephalography; epilepsy; epileptogenic tissue; neuromorphic system; spiking neural networks; system-on-a-chip.
Copyright © 2022 Burelo, Sharifshazileh, Indiveri and Sarnthein.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
Publication types
LinkOut - more resources
Full Text Sources

