General Principles Underpinning Amyloid Structure
- PMID: 35720732
- PMCID: PMC9201691
- DOI: 10.3389/fnins.2022.878869
General Principles Underpinning Amyloid Structure
Abstract
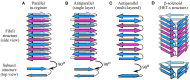
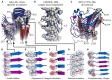
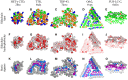
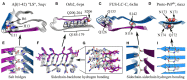
Amyloid fibrils are a pathologically and functionally relevant state of protein folding, which is generally accessible to polypeptide chains and differs fundamentally from the globular state in terms of molecular symmetry, long-range conformational order, and supramolecular scale. Although amyloid structures are challenging to study, recent developments in techniques such as cryo-EM, solid-state NMR, and AFM have led to an explosion of information about the molecular and supramolecular organization of these assemblies. With these rapid advances, it is now possible to assess the prevalence and significance of proposed general structural features in the context of a diverse body of high-resolution models, and develop a unified view of the principles that control amyloid formation and give rise to their unique properties. Here, we show that, despite system-specific differences, there is a remarkable degree of commonality in both the structural motifs that amyloids adopt and the underlying principles responsible for them. We argue that the inherent geometric differences between amyloids and globular proteins shift the balance of stabilizing forces, predisposing amyloids to distinct molecular interaction motifs with a particular tendency for massive, lattice-like networks of mutually supporting interactions. This general property unites previously characterized structural features such as steric and polar zippers, and contributes to the long-range molecular order that gives amyloids many of their unique properties. The shared features of amyloid structures support the existence of shared structure-activity principles that explain their self-assembly, function, and pathogenesis, and instill hope in efforts to develop broad-spectrum modifiers of amyloid function and pathology.
Keywords: amide ladder; amyloid structure; cryo-EM; neurodegeneration; protein aggregation; protein folding; ssNMR; steric zipper.
Copyright © 2022 Taylor and Staniforth.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures







References
-
- Adamcik J., Mezzenga R. (2011). Adjustable twisting periodic pitch of amyloid fibrils. Soft Matter. 7, 5437–5443. 10.1039/c1sm05382e - DOI
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

