Synthesis of MeOH and DME From CO2 Hydrogenation Over Commercial and Modified Catalysts
- PMID: 35720988
- PMCID: PMC9203738
- DOI: 10.3389/fchem.2022.903053
Synthesis of MeOH and DME From CO2 Hydrogenation Over Commercial and Modified Catalysts
Abstract
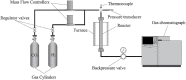
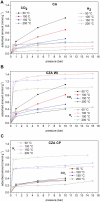
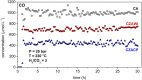
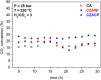
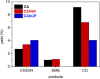
Growing concern about climate change has been driving the search for solutions to mitigate greenhouse gas emissions. In this context, carbon capture and utilization (CCU) technologies have been proposed and developed as a way of giving CO2 a sustainable and economically viable destination. An interesting approach is the conversion of CO2 into valuable chemicals, such as methanol (MeOH) and dimethyl ether (DME), by means of catalytic hydrogenation on Cu-, Zn-, and Al-based catalysts. In this work, three catalysts were tested for the synthesis of MeOH and DME from CO2 using a single fixed-bed reactor. The first one was a commercial CuO/γ-Al2O3; the second one was CuO-ZnO/γ-Al2O3, obtained via incipient wetness impregnation of the first catalyst with an aqueous solution of zinc acetate; and the third one was a CZA catalyst obtained by the coprecipitation method. The samples were characterized by XRD, XRF, and N2 adsorption isotherms. The hydrogenation of CO2 was performed at 25 bar, 230°C, with a H2:CO2 ratio of 3 and space velocity of 1,200 ml (g cat · h)-1 in order to assess the potential of these catalysts in the conversion of CO2 to methanol and dimethyl ether. The catalyst activity was correlated to the adsorption isotherms of each reactant. The main results show that the highest CO2 conversion and the best yield of methanol are obtained with the CZACP catalyst, very likely due to its higher adsorption capacity of H2. In addition, although the presence of zinc oxide reduces the textural properties of the porous catalyst, CZAWI showed higher CO2 conversion than commercial catalyst CuO/γ-Al2O3.
Keywords: CO2; DME; catalysis; fixed bed; methanol.
Copyright © 2022 Santiago, Coelho, Lucena, Musse, Portilho, Rodriguez-Castellón, Azevedo and Bastos-Neto.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures








References
-
- Aboul-Fotouh S. M. K. (2014). Production of Dimethylether (DME) as a Clean Fuel Using Sonochemically Prepared CuO And/or ZnO-Modified γ -alumina Catalysts. J. Fuel Chem. Technol. 42, 3. 10.1016/S1872-5813(14)60020-7 - DOI
-
- Adnan M. A., Kibria M. G. (2020). Comparative Techno-Economic and Life-Cycle Assessment of Power-To-Methanol Synthesis Pathways. Appl. Energy 278, 115614. 10.1016/j.apenergy.2020.115614 - DOI
-
- Afshar Taromi A., Kaliaguine S. (2018). Green Diesel Production via Continuous Hydrotreatment of Triglycerides over Mesostructured γ-alumina Supported NiMo/CoMo Catalysts. Fuel Process. Technol. 171, 20–30. 10.1016/j.fuproc.2017.10.024 - DOI
-
- Aguayo A. T., Ereña J., Mier D., Arandes J. M., Olazar M., Bilbao J. (2007). Kinetic Modeling of Dimethyl Ether Synthesis in a Single Step on a CuO−ZnO−Al2O3/γ-Al2O3 Catalyst. Ind. Eng. Chem. Res. 46, 5522–5530. 10.1021/ie070269s - DOI
-
- Ahouari H., Soualah A., Le Valant A., Pinard L., Magnoux P., Pouilloux Y. (2013). Methanol Synthesis from CO2 Hydrogenation over Copper Based Catalysts. Reac Kinet. Mech. Cat. 110, 131–145. 10.1007/s11144-013-0587-9 - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

