Dynamics of the Zebrafish Skeleton in Three Dimensions During Juvenile and Adult Development
- PMID: 35721557
- PMCID: PMC9204358
- DOI: 10.3389/fphys.2022.875866
Dynamics of the Zebrafish Skeleton in Three Dimensions During Juvenile and Adult Development
Abstract
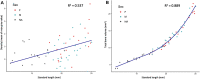
Zebrafish are a valuable model for normal vertebrate skeletogenesis and the study of myriad bone disorders. Bones grow, ossify and change shape throughout the zebrafish lifetime, and 3D technologies allow us to examine skeletogenic processes in detail through late developmental stages. To facilitate analysis of shape, orientation and tissue density of skeletal elements throughout ontogeny and adulthood, we generated a high-resolution skeletal reference dataset of wild-type zebrafish development. Using microCT technology, we produced 3D models of the skeletons of individuals ranging from 12 to 25 mm standard length (SL). We analyzed the dynamics of skeletal density and volume as they increase during juvenile and adult growth. Our resource allows anatomical comparisons between meristic units within an individual-e.g., we show that the vertebral canal width increases posteriorly along the spine. Further, structures may be compared between individuals at different body sizes: we highlight the shape changes that the lower jaw undergoes as fish mature from juvenile to adult. We show that even reproductively mature adult zebrafish (17-25 mm SL) continue to undergo substantial changes in skeletal morphology and composition with continued adult growth. We provide a segmented model of the adult skull and a series of interactive 3D PDFs at a range of key stages. These resources allow changes in the skeleton to be assessed quantitatively and qualitatively through late stages of development, and can serve as anatomical references for both research and education.
Keywords: juvenile development; microcomputed tomographic (micro-CT); skeletal anatomy; skeletogenesis; zebrafish.
Copyright © 2022 Nguyen, Lanni, Xu, Michaelson and McMenamin.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
-
- Bensimon-Brito A., Cardeira J., Dionísio G., Huysseune A., Cancela M. L., Witten P. E. (2016). Revisiting In Vivo Staining with Alizarin Red S - A Valuable Approach to Analyse Zebrafish Skeletal Mineralization during Development and Regeneration. BMC Dev. Biol. 16, 1–10. 10.1186/s12861-016-0102-4 - DOI - PMC - PubMed
-
- Boyer D. M., Gunnell G. F., Kaufman S., McGeary T. M. (2016). Morphosource: Archiving and Sharing 3-D Digital Specimen Data. Paleontol. Soc. Pap. 22, 157–181. 10.1017/scs.2017.13 - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

