The Regulation of Adipose Tissue Health by Estrogens
- PMID: 35721736
- PMCID: PMC9204494
- DOI: 10.3389/fendo.2022.889923
The Regulation of Adipose Tissue Health by Estrogens
Abstract
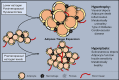
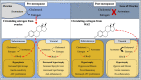
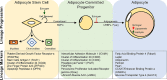
Obesity and its' associated metabolic diseases such as type 2 diabetes and cardiometabolic disorders are significant health problems confronting many countries. A major driver for developing obesity and metabolic dysfunction is the uncontrolled expansion of white adipose tissue (WAT). Specifically, the pathophysiological expansion of visceral WAT is often associated with metabolic dysfunction due to changes in adipokine secretion profiles, reduced vascularization, increased fibrosis, and enrichment of pro-inflammatory immune cells. A critical determinate of body fat distribution and WAT health is the sex steroid estrogen. The bioavailability of estrogen appears to favor metabolically healthy subcutaneous fat over visceral fat growth while protecting against changes in metabolic dysfunction. Our review will focus on the role of estrogen on body fat partitioning, WAT homeostasis, adipogenesis, adipocyte progenitor cell (APC) function, and thermogenesis to control WAT health and systemic metabolism.
Keywords: adipocyte progenitor cells; adipokines; estrogen; estrogen receptor; hypertrophy; metabolically healthy; ovariectomy; white adipose tissue.
Copyright © 2022 Steiner and Berry.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Comment in
-
Editorial: Estrogen receptors in endocrine disorders.Front Endocrinol (Lausanne). 2023 Nov 1;14:1325371. doi: 10.3389/fendo.2023.1325371. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 38027157 Free PMC article. No abstract available.
References
-
- WHO . Obesity and Overweight. World Health Organ (2013).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

