Advances in medical treatment for pancreatic neuroendocrine neoplasms
- PMID: 35721885
- PMCID: PMC9157622
- DOI: 10.3748/wjg.v28.i20.2163
Advances in medical treatment for pancreatic neuroendocrine neoplasms
Abstract
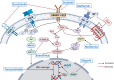
Pancreatic neuroendocrine neoplasms (PanNENs) are rare neoplasms with strong heterogeneity that have experienced an increasing incidence rate in recent years. For patients with locally advanced or distant metastatic PanNENs, systemic treatment options vary due to the different differentiations, grades and stages. The available options for systemic therapy include somatostatin analogs, mole-cularly targeted agents, cytotoxic chemotherapeutic agents, immune checkpoint inhibitors, and peptide receptor radionuclide therapy. In addition, the development of novel molecularly targeted agents is currently in progress. The sequence of selection between different chemotherapy regimens has been of great interest, and resistance to chemotherapeutic agents is the major limitation in their clinical application. Novel agents and high-level clinical evidence continue to emerge in the field of antiangiogenic agents. Peptide receptor radionuclide therapy is increasingly employed for the treatment of advanced neuroendocrine tumors, and greater therapeutic efficacy may be achieved by emerging radio-labeled peptides. Since immune checkpoint inhibitor monotherapies for PanNENs appear to have limited antitumor activity, dual immune checkpoint inhibitor therapies or combinations of antiangiogenic therapies and immune checkpoint inhibitors have been applied in the clinic to improve clinical efficacy. Combining the use of a variety of agents with different mechanisms of action provides new possibilities for clinical treatments. In the future, the study of systemic therapies will continue to focus on the screening of the optimal benefit population and the selection of the best treatment sequence strategy with the aim of truly achieving individualized precise treatment of PanNENs.
Keywords: Advanced neuroendocrine tumors; Advances; Medical treatment; Pancreatic neuroendocrine neoplasms; Peptide receptor radionuclide therapy.
©The Author(s) 2022. Published by Baishideng Publishing Group Inc. All rights reserved.
Conflict of interest statement
Conflict-of-interest statement: Authors declare no conflict of interests for this article.
Figures

References
-
- Falconi M, Eriksson B, Kaltsas G, Bartsch DK, Capdevila J, Caplin M, Kos-Kudla B, Kwekkeboom D, Rindi G, Klöppel G, Reed N, Kianmanesh R, Jensen RT Vienna Consensus Conference participants. ENETS Consensus Guidelines Update for the Management of Patients with Functional Pancreatic Neuroendocrine Tumors and Non-Functional Pancreatic Neuroendocrine Tumors. Neuroendocrinology. 2016;103:153–171. - PMC - PubMed
-
- Wu W, Jin G, Li H, Miao Y, Wang C, Liang T, Ou J, Zhao Y, Yuan C, Li Y, Lou W, Wu Z, Qin R, Wang H, Hao J, Yu X, Huang H, Tan G, Liu X, Xu K, Wang L, Yang Y, Hao C, Wang W, Guo K, Wei J, Wang Y, Peng C, Wang X, Cai S, Jiang J, Wu X, Li F, Pancreatic SSGO. The current surgical treatment of pancreatic neuroendocrine neoplasms in China: a national wide cross-sectional study. J Pancreatol. 2019;2:35–42.
-
- Ito T, Igarashi H, Nakamura K, Sasano H, Okusaka T, Takano K, Komoto I, Tanaka M, Imamura M, Jensen RT, Takayanagi R, Shimatsu A. Epidemiological trends of pancreatic and gastrointestinal neuroendocrine tumors in Japan: a nationwide survey analysis. J Gastroenterol. 2015;50:58–64. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

