IRMPD Spectroscopy of Bare Monodeprotonated Genistein, an Antioxidant Flavonoid
- PMID: 35721943
- PMCID: PMC9202291
- DOI: 10.1021/acsomega.2c01236
IRMPD Spectroscopy of Bare Monodeprotonated Genistein, an Antioxidant Flavonoid
Abstract
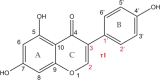
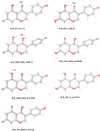
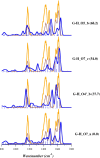
Genistein is a naturally occurring polyphenol belonging to the family of flavonoids with estrogenic properties and proven antioxidant, anti-inflammatory, and hormonal effects. Genistein and its derivatives are involved in radical scavenging activity by way of mechanisms based on sequential proton-loss electron transfer. In view of this role, a detailed structural characterization of its bare deprotonated form, [geni-H]-, generated by electrospray ionization, has been performed by tandem mass spectrometry and infrared multiple photon dissociation (IRMPD) spectroscopy in the 800-1800 cm-1 spectral range. Quantum chemical calculations at the B3LYP/6-311+G(d,p) level of theory were carried out to determine geometries, thermochemical data, and anharmonic vibrational properties of low-lying isomers, enabling to interpret the experimental spectrum. Evidence is gathered that the conjugate base of genistein exists as a single isomeric form, which is deprotonated at the most acidic site (7-OH) and benefits from a strong intramolecular H-bond interaction between 5-OH and the adjacent carbonyl oxygen in the most stable arrangement.
© 2022 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
-
- Sychrová A.; Koláriková I.; Žemlička M.; Šmejkal K. Natural Compounds with Dual Antimicrobial and Anti-Inflammatory Effects. Phytochem. Rev. 2020, 19, 1471–1502. 10.1007/s11101-020-09694-5. - DOI
LinkOut - more resources
Full Text Sources

